Brain Immune Responses and Cell Death

Next, I will explaine a pathway by which reactive oxygen species (ROS) activate immune responses and trigger inflammation in the brain, inducing neurodegeneration.
Neurodegeneration is a process which leads to irreversible neuronal damage and death. (Giacalone et al. 2015)
I wiil then present studies showing that EMFs did indeed induce neurodegeneration from brain immune responses.
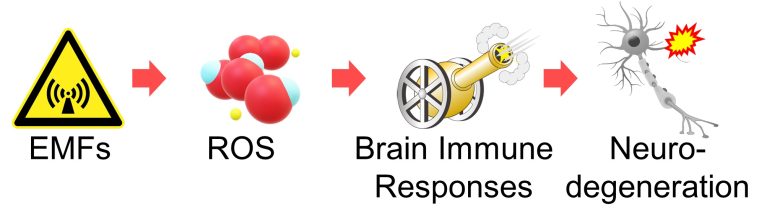
Table of ContentsAll_Pages
Brain Immune Responses and Neuroinflammation
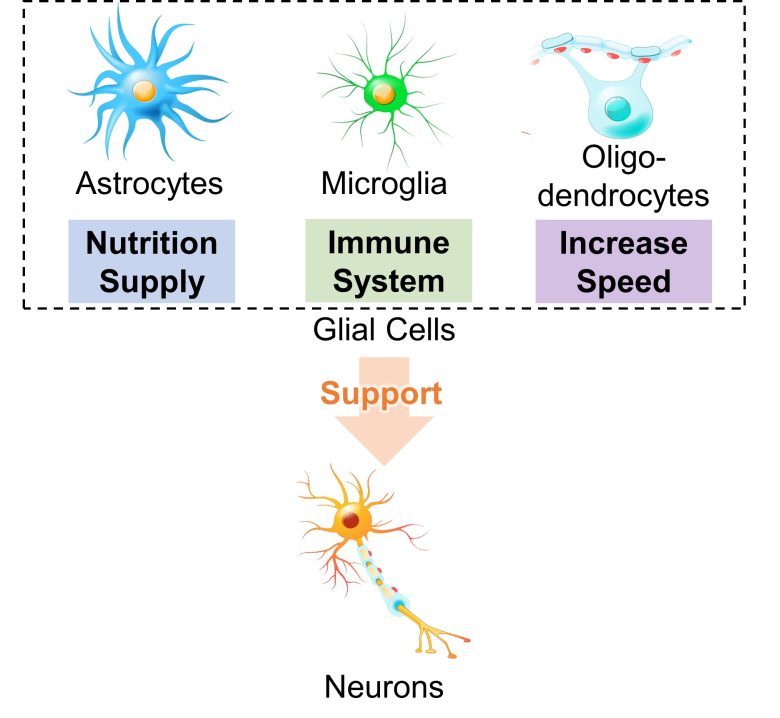
Information processing and transmission in the brain are handled by brain cells called neurons.
In addition, other brain cells include astrocytes, which supply nutrients to neurons; microglia, which serve as brain immune system; and oligodendrocytes, which increase the speed of neurotransmission, all of which support the activity of neurons.
These brain cells are called glial cells.
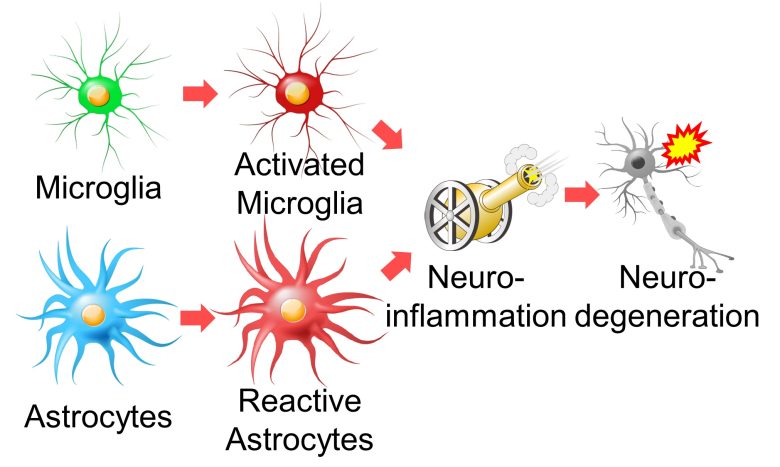
Besides microglia, astrocytes are also responsible for immune responses in the brain and are activated in response to various lesions.
Particularly activated astrocytes are called reactive astrocytes, and their morphology and function are altered to a state different from that of normal astrocytes. (Ben Haim et al. 2015)
Inflammation in the brain is called neuroinflammation, and it is known that the immune response of these glial cells causes neuroinflammation and is involved in various neurodegenerative diseases. (Heneka et al. 2014)
Brain Immune Responses due to ROS
ROS are known to activate immune cells and cause inflammation. (Chatterjee 2016)
NF-κB is a protein that regulates various aspect of immune responses (Hayden et al. 2006), and ROS is known to activate this NF-κB (Gloire et al. 2006).
For example, ROS activate immune cell macrophages via activation of NF-κB. (Rendra et al. 2019)
Also in the nervous system, ROS activate microglia, which are resident macrophages in the brain, via activation of NF-κB. (Bordt and Polster 2014)
Furthermore, it has been confirmed that activated microglia release inflammation-promoting substances called pro-inflammatory cytokines into the surroundings, which in turn change astrocytes into reactive astrocytes. (Liddelow et al. 2017)
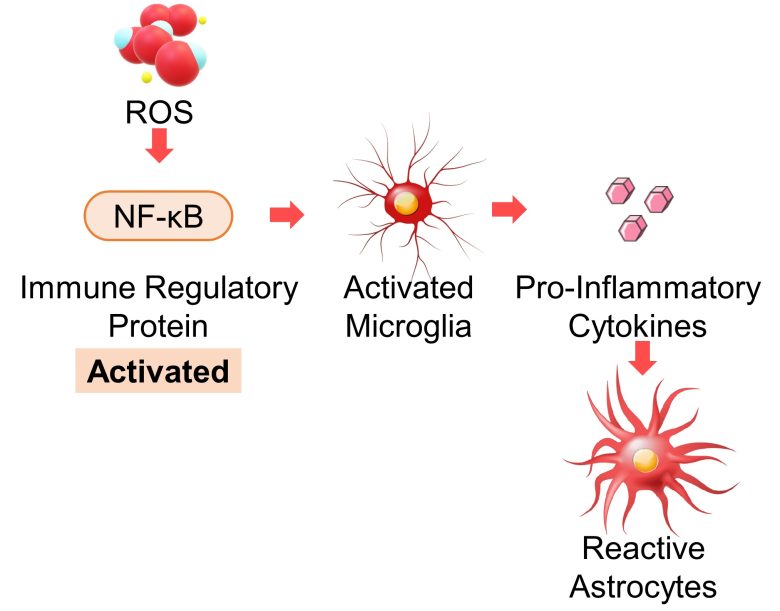
In this way, microglia and astrocytes activated by ROS cause neuroinflammation.
Astrocyte Neuroinflammation
Neuroinflammation caused by reactive astrocytes (activated astrocytes), leads to neurodegeneration.
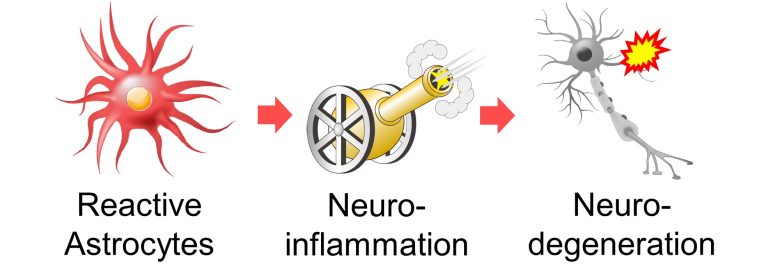
A 2015 France paper provides a comprehensive description of the role of reactive astrocytes in neurodegenerative diseases. (Ben Haim et al. 2015)
Based on this paper, I will present the following neurodegenerative pathways due to reactive astrocytes.
- Decreased supply of nutrients and antioxidants
- Induction into excitotoxicity
- Increased release of gliotransmitters
- Massive production of amyloid-β
Decreased Supply of Nutrients and Antioxidants
Blood vessels in the brain are sealed by the blood–brain barrier, so neurons need to be supplied with nutrients from astrocytes.
These nutrients include glucose, lactic acid (lactate), cholesterol, and antioxidants. (Ben Haim et al. 2015)
Lactic acid is an important source of energy for the brain (van Hall et al. 2009), cholesterol is a component of cell membranes and necessary for synapse formation, and antioxidants are necessary for protection against oxidative stress.
However, when astrocytes turn into reactive astrocytes, the supply of these nutrients is reduced (Ben Haim et al. 2015), which can be detrimental to neurons.
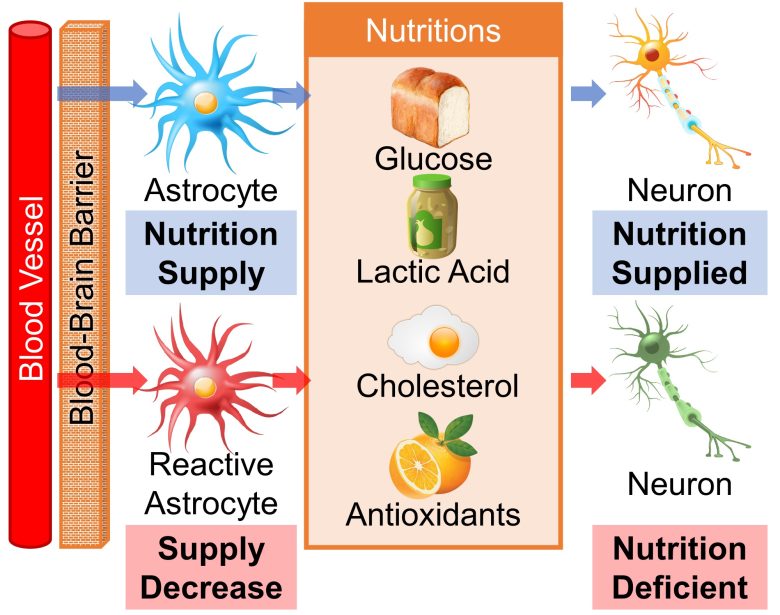
Induction into Excitotoxicity
Neuronal Excitation
When a neuron is stimulated, it generate an electrical signal called an action potential, which travels through within the neuron.
The state where action potentials are generated is called "excitation".
When the action potential reaches the cell terminal, the neuron releases a chemical signal called neurotransmitters, which excite a neighboring neuron.
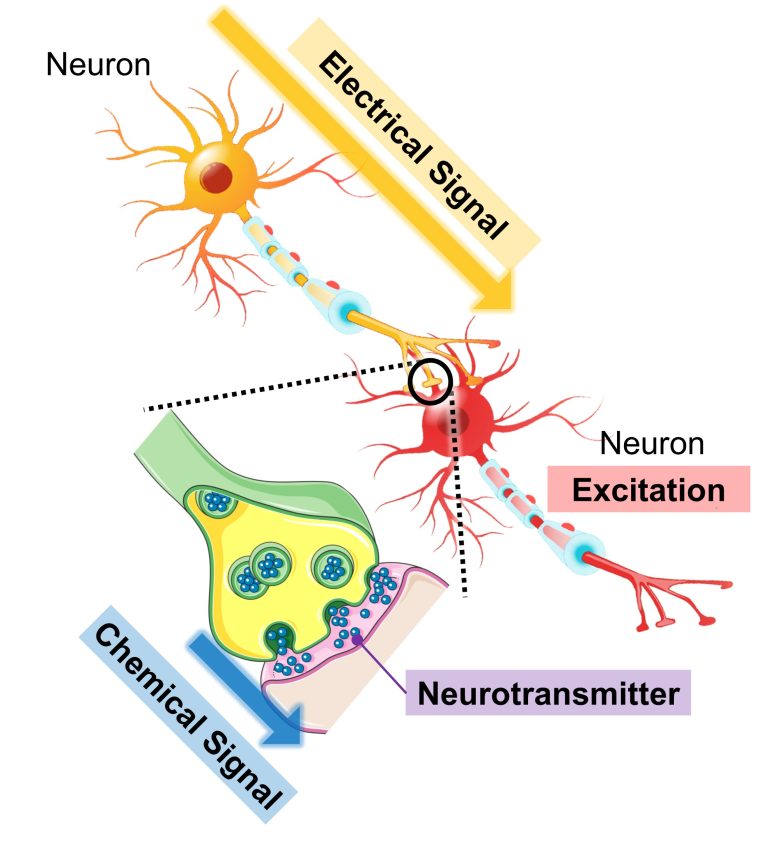
This in turn generates an action potential in the neighboring neuron, and when it travels to the cell terminal, it releases neurotransmitters as well, and the information is transmitted to the subsequent neuron.
Successions of many neurons connected in this way forms the circuitry for information processing and transmission in the brain.
Excitotoxicity by Astrocytes
Several types of neurotransmitters exist, including glutamic acid (glutamate), dopamine, serotonin, and GABA.
Here, astrocytes are responsible for the uptake of glutamic acid, preventing excitotoxicity, which leads to neuronal cell death due to continued neuronal excitation. (Ben Haim et al. 2015)
However, when astrocytes turn into reactive astrocytes, the uptake becomes insufficient, and neurons fall into excitotoxicity. (Ben Haim et al. 2015)
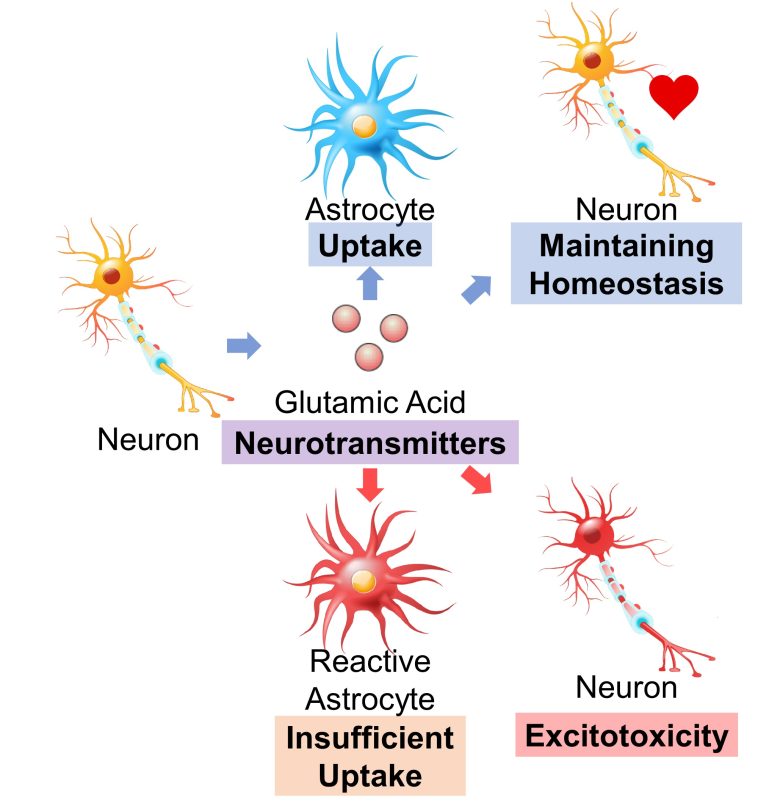
Excitotoxicity is known to be observed in numerous neurodegenerative diseases, including Huntington's disease, Alzheimer's diseases, ALS, Parkinson's disease, and multiple sclerosis. (Salińska et al. 2005)
Increased Release of Gliotransmitters
It is gaining evidence that astrocytes, like neurons, bind to each other for information transmission, and that the release of gliotransmitters, not neurotransmitters, excites not only the astrocytes but also the neurons. (Scemes and Giaume 2006)
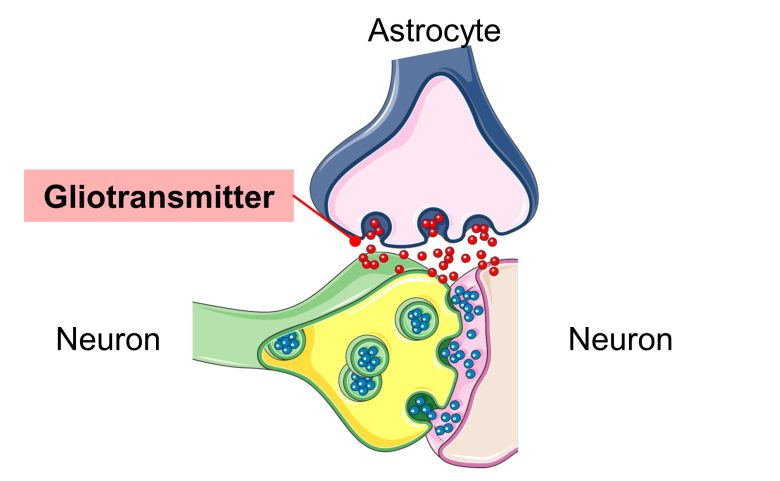
Known gliotransmitters include glutamic acid, ATP, and GABA. (Ben Haim et al. 2015)
ATP is well known as a source of cellular energy, but surprisingly, it also acts as a gliotransmitter and activates neurons and astrocytes. (Butt 2011)
And when astrocytes turn into reactive astrocytes, the release of these gliotransmitters increases, causing excitotoxicity in neurons. (Ben Haim et al. 2015, Ding et al. 2007)
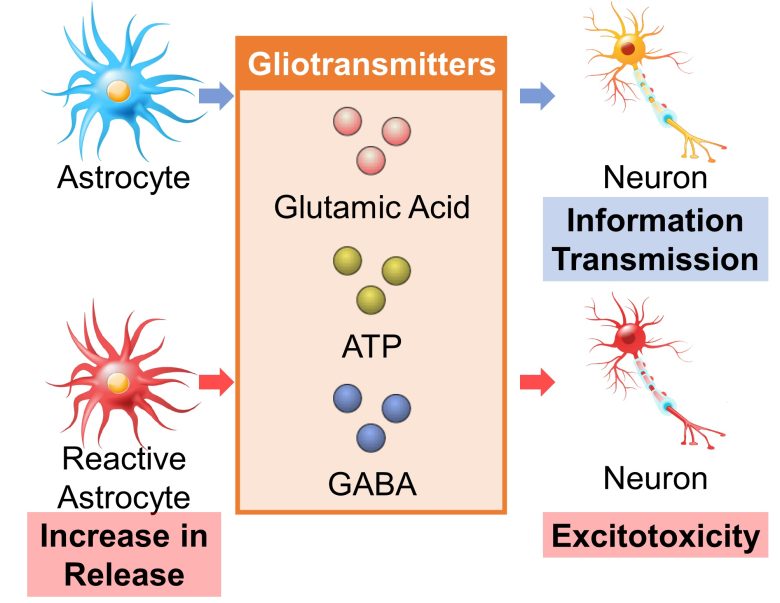
GABA is a type of neurotransmitter that inhibits excitation, but its increased release from reactive astrocytes has been shown to inhibit the growth of hippocampal synapses in mice, causing learning and memory deficits. (Ben Haim et al. 2015, Jo et al. 2014, Wu et al. 2014)
Massive Production of Amyloid-β
Aggregation of intra- or extra-cellular proteins is a central feature of neurodegenarative diseases. (Ben Haim et al. 2015)
For example, in Alzheimer's disease, a protein called amyloid-β aggregates in the extra-cellular space and forms a deposit called a senile plaque.
This senile plaque is believed to be neurotoxic.
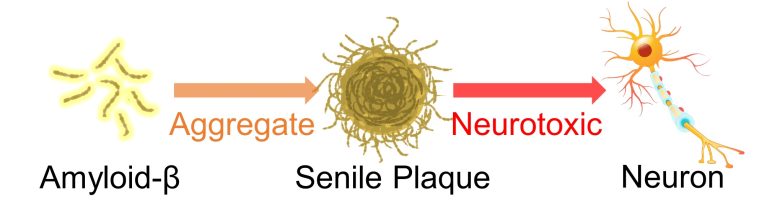
The synaptic activity of neurons generates this amyloid-β and releases it into the extra-cellular space (Cirrito et al. 2005), and this is cleared by astrocytes (Ben Haim et al. 2015).
However, it has been confirmed that when they turn into reactive astrocytes, they conversely begin to produce massive amounts of amyloid-β. (Ben Haim et al. 2015, Zhao et al. 2011)
Senile plaques also activate astrocytes, inducing them to chronically produce amyloid-β. (Hu and Van Eldik 1999)
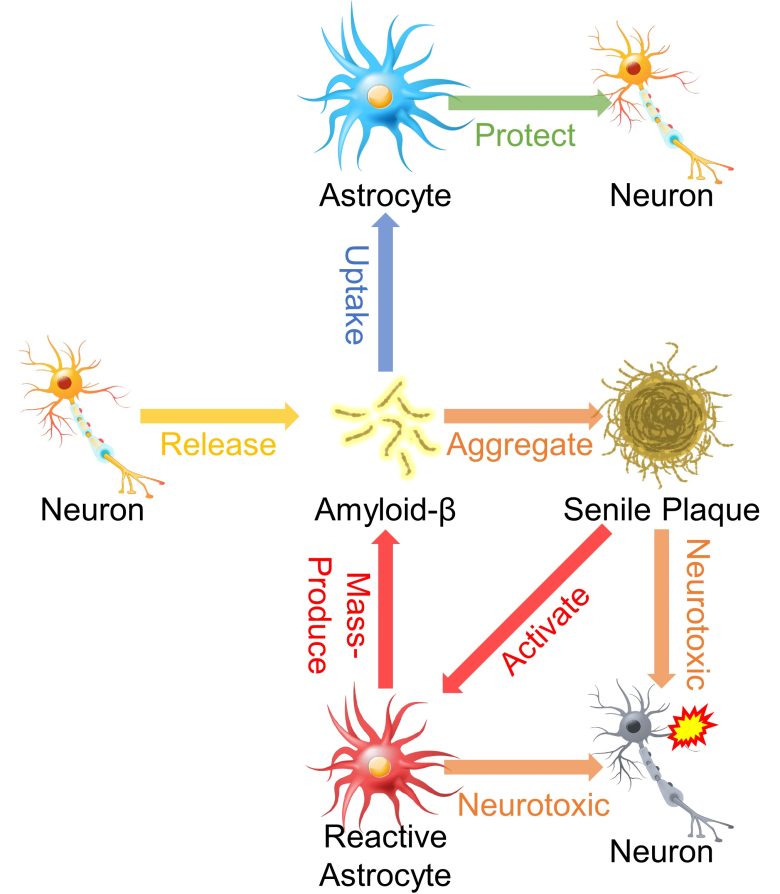
These chronically activated astrocytes, along with senile plaques, are likely to cause neurotoxicity.
Microglia Neuroinflammation
Neuroinflammation caused by activated microglia also leads to neurodegeneration.
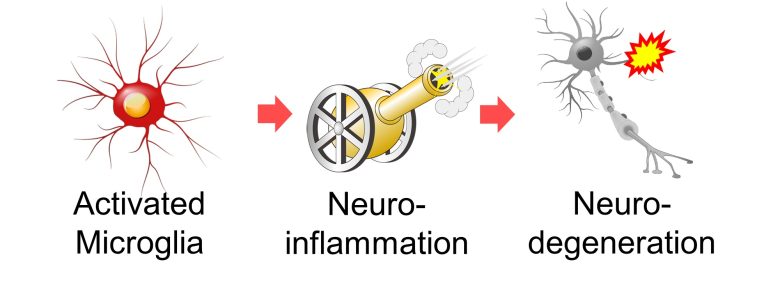
Inflammation Collateral Damage
When microglia are activated, proteins called NADPH oxidase and nitric oxide synthase are deployed at the cell membrane. (Brown 2007)
These produce superoxide and nitric oxide, respectively, and these two substances react to form peroxynitrite. (Brown 2007)
Peroxynitrite has anti-viral, anti-microbial, and anti-parasitic effects (Ascenzi et al. 2010), but neurons suffer the collateral damage, leading to cell death. (Brown 2007)
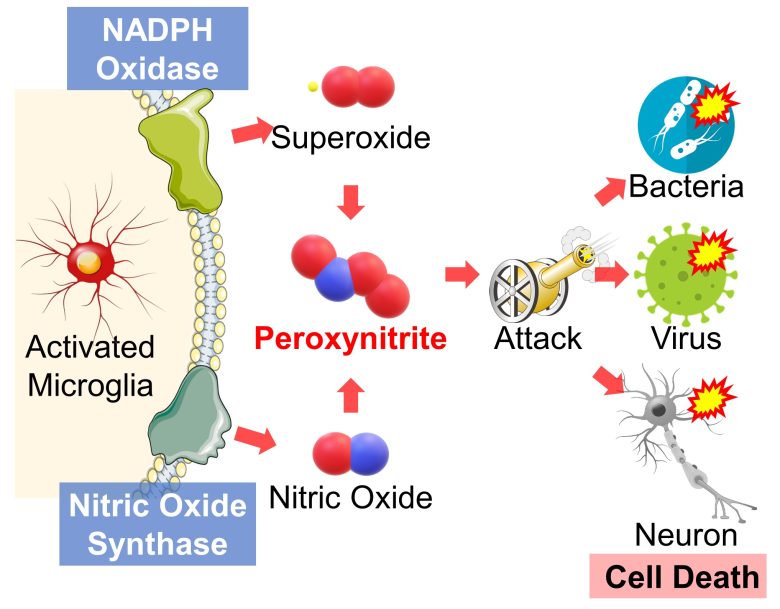
Reactive astrocytes may also be involved in the neuronal cell death due to this peroxynitrite production. (Ben Haim et al. 2015)
Neurodegeneration due to Brain Immune Responses
I will wrap up the topic.
Increased ROS in the brain activates immune cell microglia, which in turn activate astrocytes.
The immune responses of these cells causes inflammation, leading to neurodegeneration.
Also, it has been confirmed that EMFs increase ROS in the brain.
Therefore, we can draw the conclusion that EMFs trigger immune responses and cause inflammation in the brain, ultimately leading to neurodegeneration.
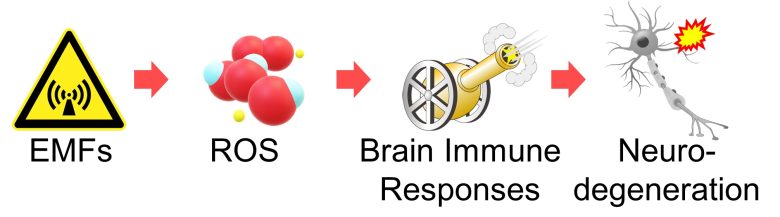
Brain Immune Responses and Neurodegeneration by EMFs
Here, I will present studies showing that bain imuune responses occured with EMF exposure, leading to neurodegeneration.
Studies
Yang et al. 2010
Mouse microglia were exposed to pulse-modulated 2.45 GHz RF-EMF at a culture-medium average SAR of 6 W/kg for only 20 minutes.
As a result, microglia were activated and the release of pro-inflammatory cytokines, signals that activate aslotocytes, increased.
Activation of Microglia
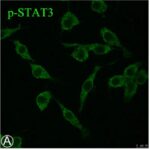
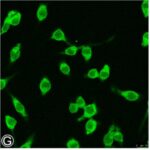
Due to the RF-EMF exposure, phosphorylation of STAT3, a marker for microglial activation, increased.
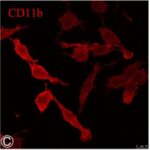
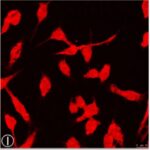
Due to the RF-EMF exposure, CD11b expression, a marker for microglial activation, increased.
Increase in Release of
Pro-Inflammatory Cytokines
The release of pro-inflammatory cytokine TNF-α increased as the time passed after RF-EMF exposure.
Megha et al. 2015
Male rats were exposed to RF-EMFs of 900 MHz, 1800 MHz, and 2450 MHz at whole-body average SARs of 0.00059 W/kg, 0.00058 W/kg, and 0.00066 W/kg, respectively, for 2 hours per day for 60 days.
As a result, in the hippocampi of the rats, ROS increased and the release of pro-inflammatory cytokines, signals that activate aslotocytes, increased as the frequency of the RF-EMFs increased.
Increase in ROS
An increase in lipid peroxidation and a decrease in antioxidant activity mean an increase in ROS.
Increase in Release of
Pro-Inflammatory Cytokines
MAUSSETBONNEFONT et al. 2004
Adult male rats were exposed to RF-EMFs similar to those emitted from cell phones at a brain average SAR of 6 W/kg for only 15 minutes.
As a result, in the brains of the rats, reactive astrocytes increased in the cortex, hippocampus, and especially in the striatum of the basal ganglia.
Increase in Reactive Astrocytes 1
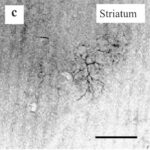
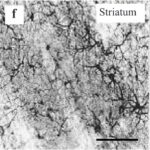
Due to only 15 minutes of the RF-EMF exposure, reactive astrocytes increased in the striatum of the basal ganglia.
Increase in Reactive Astrocytes 2
The reactive astrocytes marker increased by 25% in the cortex, by 20% in the hippocampus, and by 140% in the striatum of the basal ganglia due to only 15 minutes of the RF-EMF exposure.
Ammari et al. 2010
Male rats aged 6 weeks, equivalent to children, were exposed to RF-EMFs similar to those emitted from cell phones for 45 minutes per day at a brain average SAR of 1.5 W/kg, or 15 minutes per day at 6 W/kg, for 8 weeks.
As a result, in the brains of the rats 3 days after the exposure, reactive astrocytes increased in the prefrontal cortex, the dentate gyrus of the hippocampus, the striatum and globus pallidus of the basal ganglia, and the cerebellar cortex.
Increase in Reactive Astrocytes
Akakin et al. 2020
Cell phones with a local SAR of 1.79 W/kg were placed on top of the breeding cages and kept on standby mode or on talk mode for 2 hours per day, and pregnant rats were exposed to their EMFs for a week in early pregnancy, and then the born-pup rats were exposed for another 2 months (equivalent to infancy/childhood).
As a result, in the brains of the pup rats, reactive astrocytes increased as the output power of the cell phones increased and as the exposure period increased.
Furthermore, neurons degenerated in the cerebral cortex, hippocampal dentate gyrus, and hippocampal Ammon's horn, and the trigeminal nerves and their myelin sheath also degenerated.
Increase in Reactive Astrocytes
Neurodegeneration
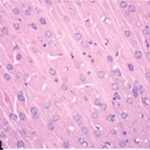
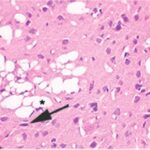
Due to the exposure to the cell phone EMFs during the fetushood, infancy, and childhood, numerous vacuoles (cavities) occurred in the cerebral cortex, and a large number of shrunken, darkly stained, degenerated neurons, which seem to be dark neurons, appeared.
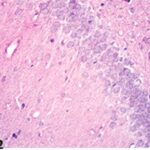
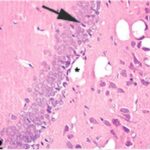
Due to the exposure to the cell phone EMFs during the fetushood, infancy, and childhood, numerous vacuoles (cavities) occurred in the hippocampal dentate gyrus, and a large number of shrunken, darkly stained, degenerated neurons, which seem to be dark neurons, appeared.
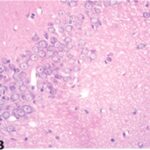
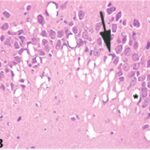
Due to the exposure to the cell phone EMFs during the fetushood, infancy, and childhood, numerous vacuoles (cavities) occurred in the hippocampal Ammon's horn, and a large number of shrunken, darkly stained, degenerated neurons, which seem to be dark neurons, appeared.
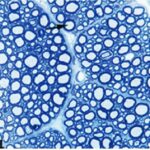
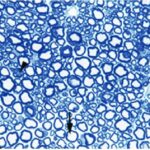
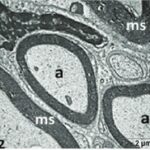
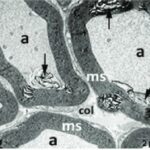
Due to the exposure to the cell phone EMFs during the fetushood, infancy, and childhood, the axons of the trigeminal nerves and their myelin sheaths were degenerated.
Afeefy et al. 2013
Cell phones with a local SAR of 0.43 W/kg were placed outside the breeding cages and kept on incoming calls for 2 hours per day, and pregnant rats were exposed to their EMFs for 3 weeks throughout the entire pregnancy, and then the born-pup rats were exposed for another 4 weeks (equivalent to infancy).
As a result, in the hippocampi of the pup rats, reactive astrocytes increased.
Furthermore, neurons degenerated in the dentate gyrus and Ammon's horn.
Increase in Reactive Astrocytes
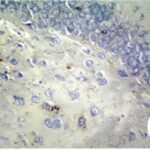
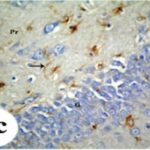
Due to the exposure to the cell phone EMFs during the fetushood and infancy, reactive astrocytes increased in the hippocampal dentate gyrus.
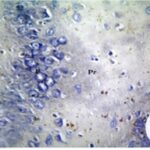
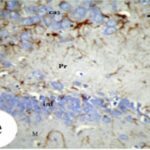
Due to the exposure to the cell phone EMFs during the fetushood and infancy, reactive astrocytes increased in the hippocampal Ammon's horn.
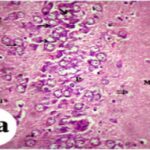
Neurodegeneration in
the Hippocampal Dentate Gyrus
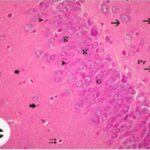
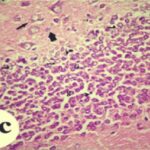
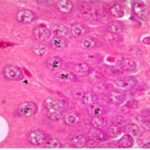
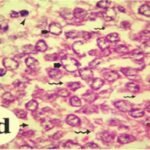
Due to the exposure to the cell phone EMFs during the fetushood and infancy, neurons (granule cells) degenerated in the hippocampal dentate gyrus.
Neurodegeneration in
the Hippocampal Ammon's Horn
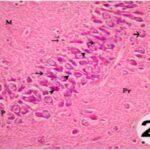
Due to the exposure to the cell phone EMFs during the fetushood and infancy, neurons (pyramidal cells) degenerated in the the hippocampal Ammon's horn.
The next experiment from the same study was conducted on adult rats.
Cell phones with a local SAR of 0.43 W/kg were placed outside the breeding cages and kept on incoming calls for 2 hours per day, and adult rats were exposed to their EMFs for 12 weeks.
As a result, in the hippocampi of the adult rats, reactive astrocytes increased.
Furthermore, neurons degenerated in the dentate gyrus and Ammon's horn.
Increase in Reactive Astrocytes
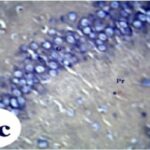
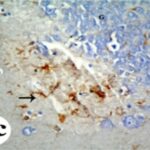
Due to the exposure to the cell phone EMFs, reactive astrocytes increased in the hippocampal dentate gyrus.
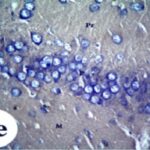
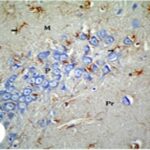
Due to the exposure to the cell phone EMFs, reactive astrocytes increased in the hippocampal Ammon's horn.
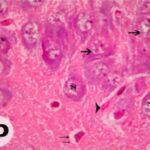
Neurodegeneration in
the Hippocampal Dentate Gyrus
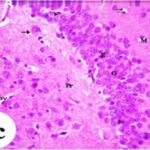
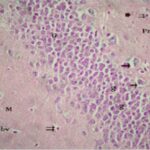
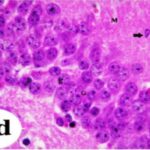
Due to the exposure to the cell phone EMFs, neurons (granule cells) degenerated in the the hippocampal dentate gyrus.
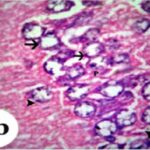
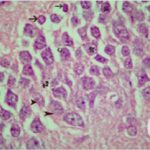
Neurodegeneration in
the Hippocampal Ammon's Horn
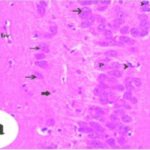
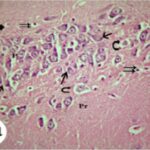
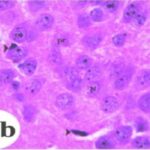
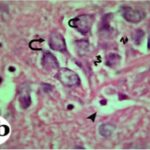
Due to the exposure to the cell phone EMFs, neurons (pyramidal cells) degenerated in the the hippocampal Ammon's horn.
That's all for the presentation of the studies.
We have confirmed that EMFs can indeed cause immune responses in the brain, leading to neurodegeneration.