Mitochondrial Damage and Cell Death
Next, I will explain a pathway by which reactive oxygen species (ROS) damage mitochondria, leading to cell death.
I will then present studies showing that EMFs did indeed induce cell death from mitochondria damage.
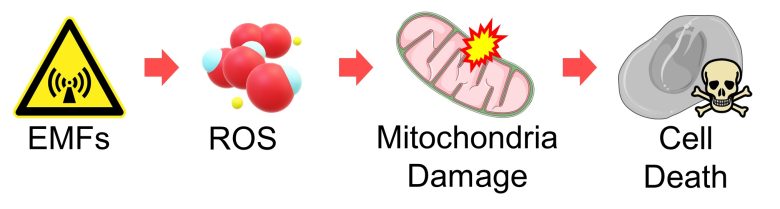
Table of ContentsAll_Pages
Mitochondrial Cellular Respiration
What is Cellular Respiration?
Respiration can be divided into the following two processes:
External Respiration
The process where living organisms take oxygen in from the air into blood and give carbon dioxide off in exchange.
Internal Respiration
The process where cells take oxygen in from blood and give carbon dioxide off in exchange.
Cellular respiration, where cells use oxygen to extract energy from nutrients and expel carbon dioxide and water, is the primary process of internal respiration.
And it is mitochondria that are responsible for this cellular respiration.
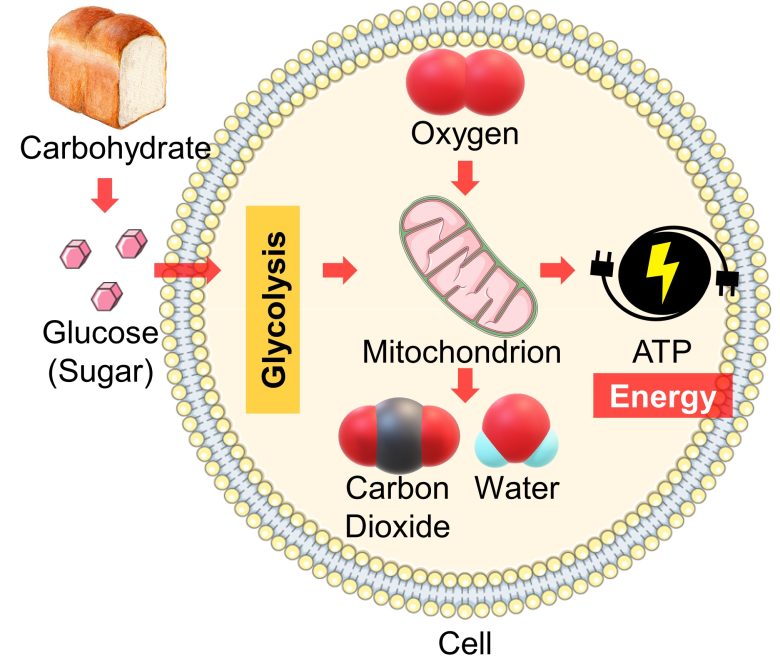
Cellular Respiration Process
When carbohydrates are digested, they are broken down to glucose (sugar), which is then distributed throughout the body via the bloodstream.
Once cells are supplied with glucose from the blood, the glucose undergoes further breakdown, called glycolysis, into the mitochondria.
Mitochondria then use oxygen to convert this into cellular energy called ATP and expel carbon dioxide and water as waste products.
Energy Extraction
Cellular respiration is divided into several processes, but here I will explain the process by which mitochondria extract energy (ATP).
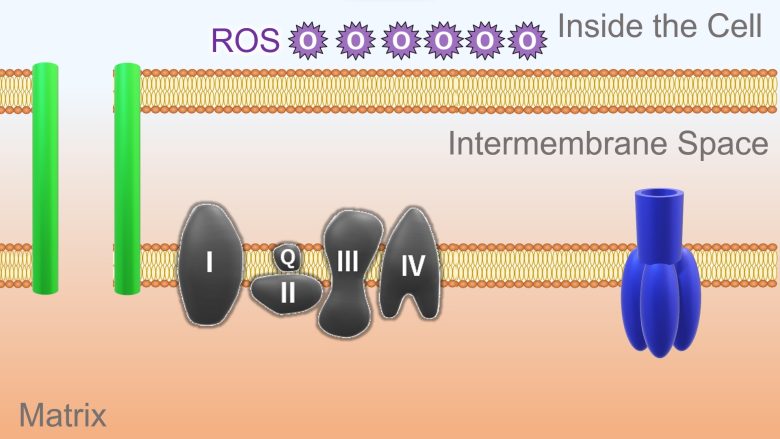
A mitochondrion is divided into an inner compartment called the matrix and an outer compartment called the intermembrane space.
On the membrane separating the compartments reside subunits called the electron transport chain and ATP synthase, which extract energy from a molecule derived from glucose.
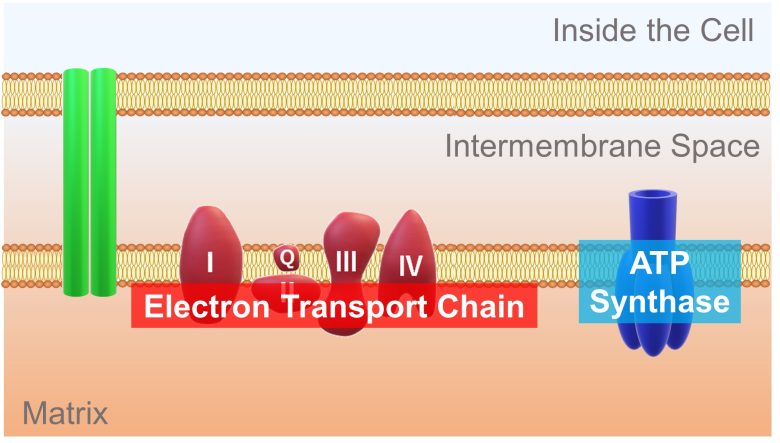
A high-energy electron is passed from the molecule derived from glucose to this electron transport chain. The electron release energy as it travel down the electron transport chain. This energy is used to pump hydrogen ions from the matrix into the intermembrane space.
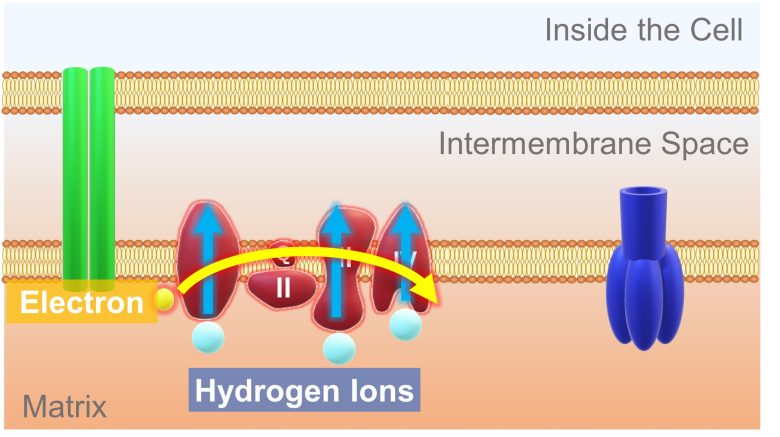
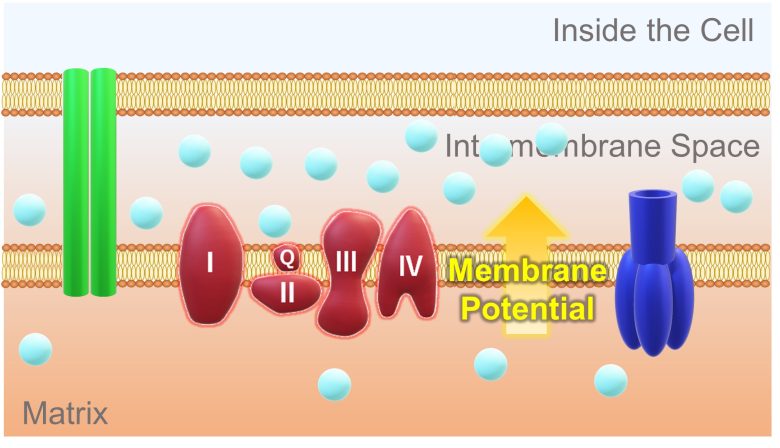
This results in more hydrogen ions in the intermembrane space and less in the matrix. Therefore, a concentration difference in hydrogen ions is generated. In addition, because hydrogen ions are positively charged, a potential difference called the membrane potential is also generated.
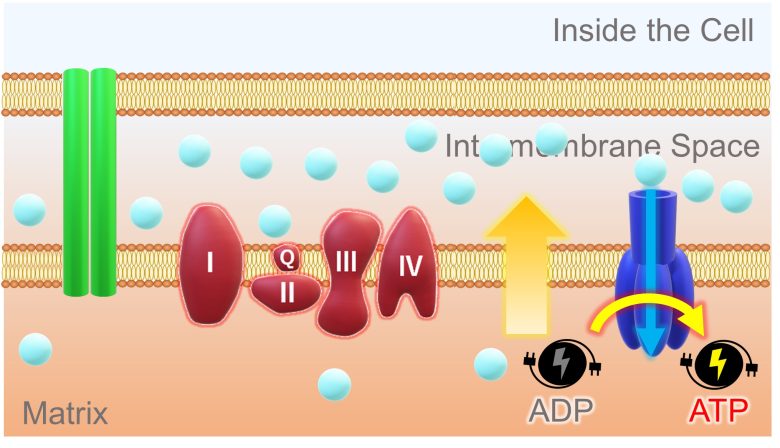
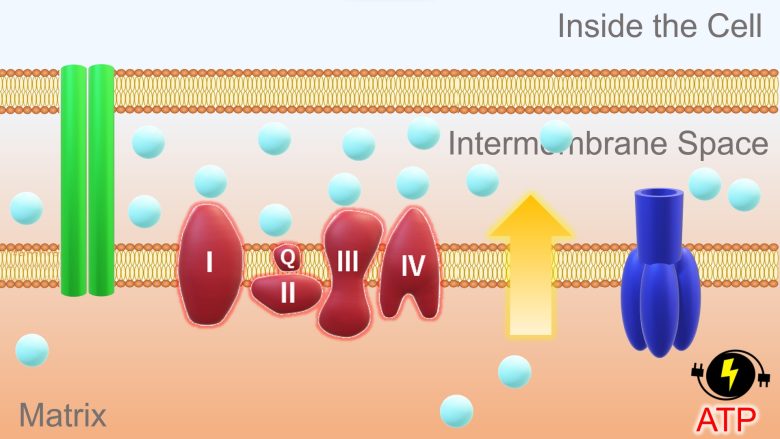
This concentration and potential difference allows hydrogen ions in the intermembrane space to diffuse through a pore of the ATP synthase and into the matrix. ATP synthase uses the energy of this back-flowing ion to synthesize ATP from ADP.
ADP is the state of ATP before energy is stored, and ATP that has expended energy returns to ADP.
ROS Amplification
Mitochondria have the nature of amplifying ROS when exposed to ROS, termed ROS-induced ROS-release. (Zorov et al. 2006)
Based on a 2006 Russia paper, I will explain the process. (Zorov et al. 2006)
First, when mitochondria are exposed to ROS, a pore called the mitochondrial membrane permeability transition pore (mPTP) opens.
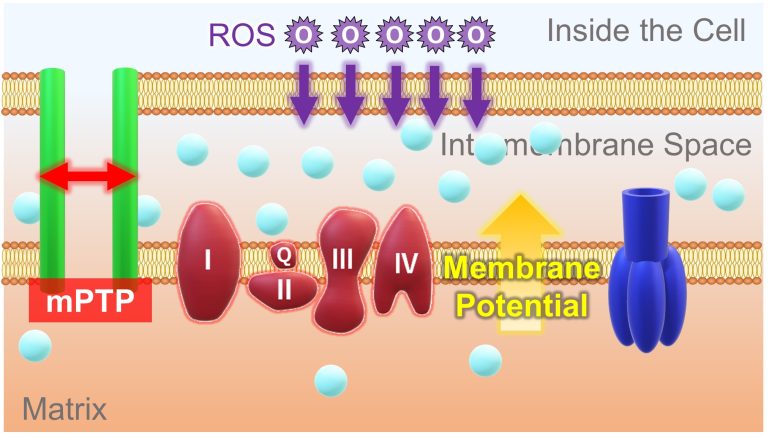
Then, a large amount of hydrogen ions flow into the matrix. With this, there is no longer a concentration difference in hydrogen ions between the intermembrane space and the matrix, resulting in the loss of membrane potential.
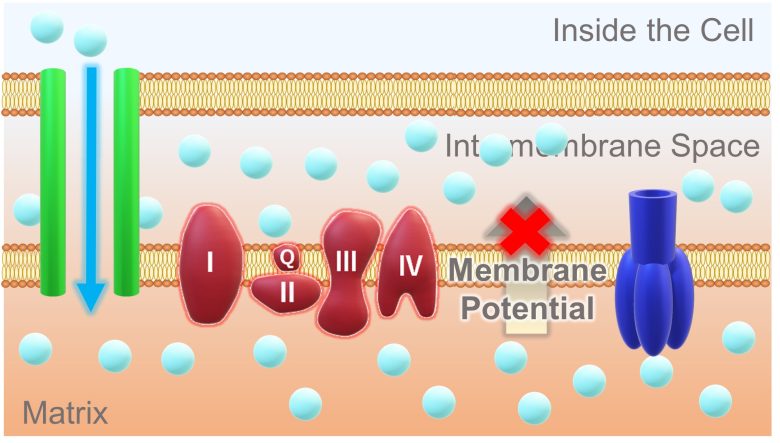
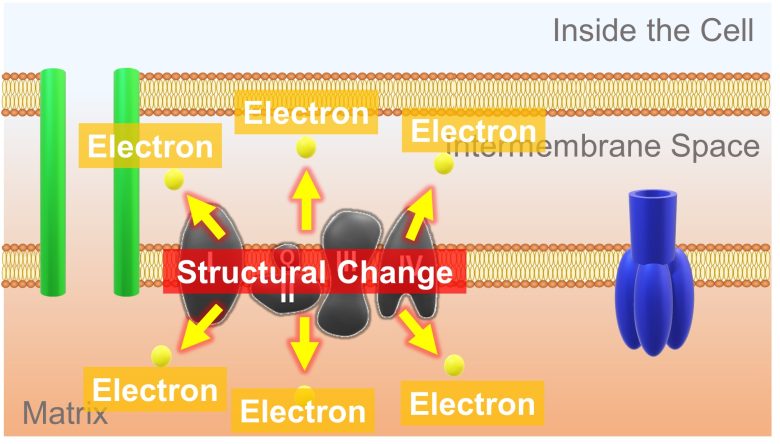
In addition, during this process, a large amount of electrons leak from the electron transport chain to the surroundings.
This is most probably due to a structural change in the electron transport chain, inhibiting the normal pathway of electron transport. (Batandier et al. 2004)
These leaked large amount of electrons combine with the oxygen present in the surroundings and turn into superoxide.
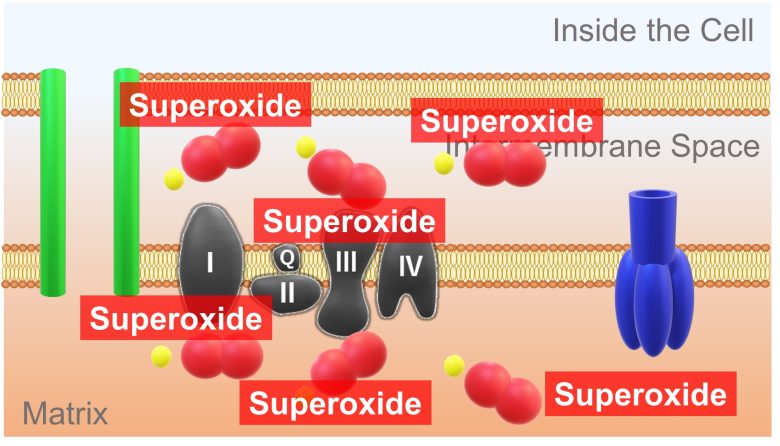
As a result, a large amount of ROS are produced.
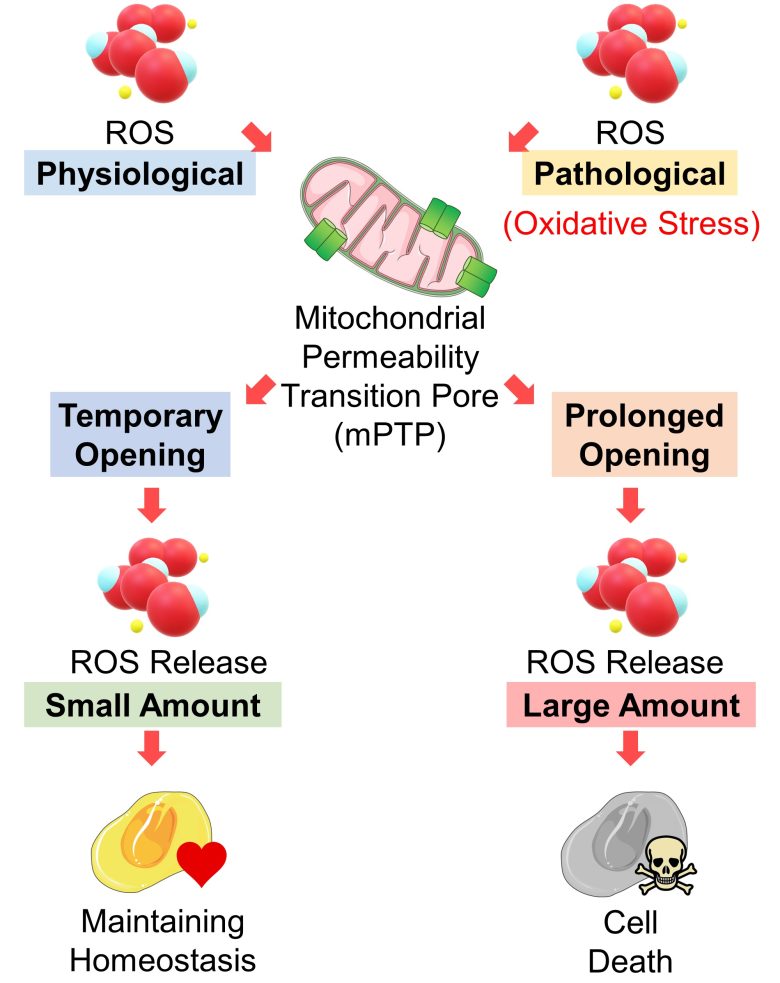
Influence of mPTP Opening
When ROS are in physiological amounts, mPTP opening is only temporary, releasing a small amount of ROS, which play a role in maintaining cellular homeostasis (stable and balanced state). (Zorov et al. 2014)
On the other hand, when ROS increase to pathological amounts, i.e., when mitochondria are exposed to oxidative stress, its opening is prolonged, releasing a large amount of ROS, which leads to cell death. (Zorov et al. 2014)
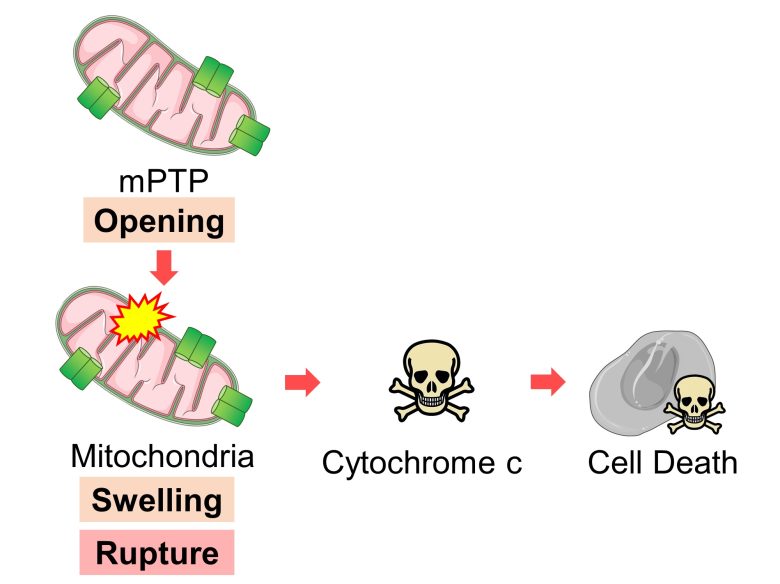
The opening of mPTP also leads to mitochondrial swelling and even rupture, releasing a signal called cytochrome c, which induces cell death. (Halestrap et al. 2000)
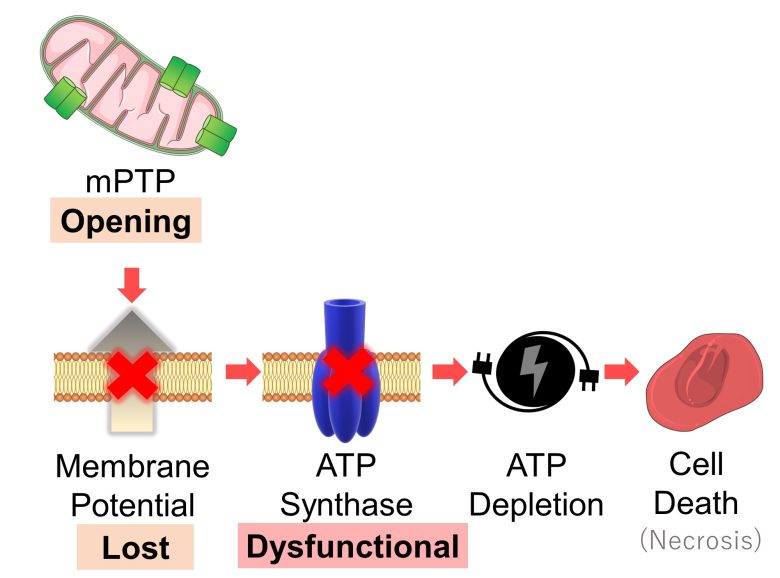
Furthermore, the opening of mPTP causes the loss of mitochondrial membrane potential, which in turn causes ATP synthase to become dysfunctional, and if this persists for a prolonged period, the cell will undergo ATP depletion, leading to cell death (necrosis). (Halestrap 2009)
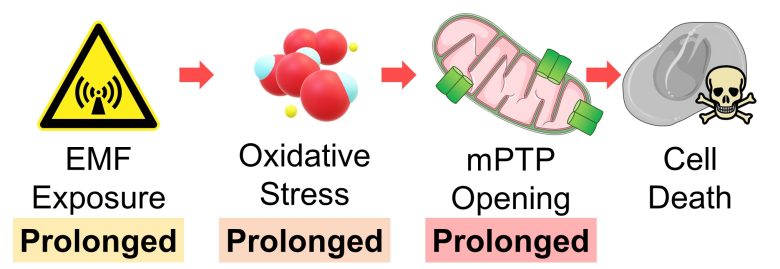
Cell Death due to Prolonged mPTP Opening
I will wrap up the topic.
Prolonged opening of mPTP induces cell death due to mitochondrial swelling and rupture, as well as ATP depletion.
Prolonged oxidative stress leads to prolonged opening of mPTP.
Also, prolonged exposure to EMFs inevitably leads to prolonged oxidative stress.
Therefore, we can draw the conclusion that cells die when exposed to EMFs over a prolonged period.
Mitochondrial Damage and Cell Death by EMFs
Here, I will present studies that showing mitochondria were damaged with EMF exposure, resulting in cell death.
Studies
Zuo et al. 2014
Neuron-like cells differenciated from the PC12 neural cell line were exposed to 2.856GHz microwaves with a strength of 30 mW/cm2 for only 5 minutes.
As a result, neuron-like cells were damaged, mitochondrial membrane potential declined, cytochrome c, a signal of cell death from mitochondria, increased, and cell death increased.
Damage to Neuron-Like Cells
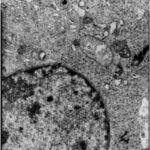
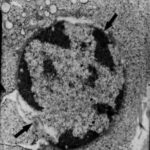
Due to the microwave exposure, neuron-like cells were damaged.
Decline in Mitochondrial Membrane Potential
The mitochondrial membrane potential marker decreased by 40% due to the microwave exposure.
Increase in Cell Death Signal
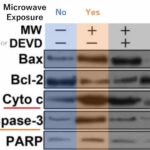
Due to the microwave exposure, cytochrome c, a signal of cell death, as well as proteins such as caspase-3, which are activated in respond to the signal, increased.
Increase in Cell Death
The percentage of cell death of neuron-like cells increased 2-fold due to the microwave exposure.
Liu et al. 2012
Rat astrocytes, which are non-neuronal cells in the brain, and rat glioma cells were exposed to 1950MHz RF-EMFs at a culture-medium average SAR of 5.36 W/kg for 48 hours
As a result, in the astrocytes, mitochondria were swelling and damaged, cell death increased and cell count decreased.
On the other hand, glioma cells, cancerous cells of astrocytes, were not largely affected by the RF-EMFs.
Damage to Mitochondria
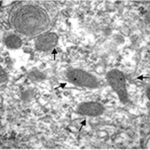
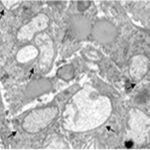
Due to the RF-EMF exposure, the mitochondria in the astrocytes were swelling and damaged.
Increase in Cell Death
Decrease in Cell Count
Kiray et al. 2012
Adult male rats were exposed to 50 Hz ELF-EMFs with a strength of 3 mT for 4 hours per day for 2 months.
As a result, ROS increased in the heart, and mitochondria were swelling and damaged and cell death increased in the heart muscle.
Increase in ROS
An increase in lipid peroxidation and a decrease in antioxidant activity mean an increase in ROS.
Damage to Mitochondria
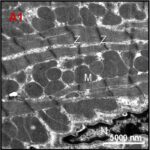
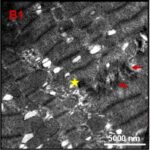
Due to the ELF-EMF exposure, mitochondria in the heart muscle were swelling and damaged.
Increase in Cell Death
Türedi et al. 2014
Pregnant rats were exposed to 900 MHz RF-EMFs with a strength of 50 μW/cm2 for 1 hour per day for 1 week in late pregnancy.
As a result, in the heart muscle of the born-son rats, mitochondria were swelling and damaged, and cell death increased.
Damage to Mitochondria
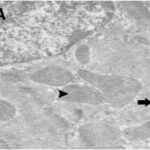

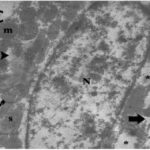
Due to the RF-EMF exposure, mitochondria in the heart muscle were swelling and damaged.
Increase in Cell Death
The percentage of cell death in the heart muscle increased 3-fold due to the RF-EMF exposure.
Liu et al. 2015
Male rats aged 8 weeks, equivalent to adolescents, were exposed to pulse-modulated 2.9 GHz RF-EMFs with a strength of 50 mW/cm2 for 6 minutes per day for 12 months.
As a result, in the sinus node of the heart, mitochondria were swelling and damaged, and parenchymal cells (functional cells) decreased.
Damage to Mitochondria
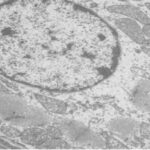
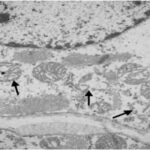
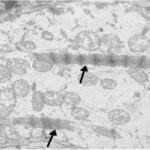
Due to the RF-EMF exposure, mitochondria in the sinus node were swelling and damaged.
Decrease in Cell Count
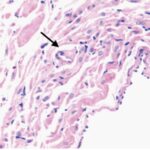
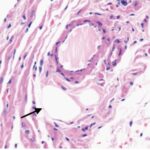
Due to the RF-EMF exposure, parenchymal cells in the sinus node decreased.
That's all for the presentation of the studies.
We have confirmed that EMFs can indeed damage mitochondria and cause cell death.


