Recap

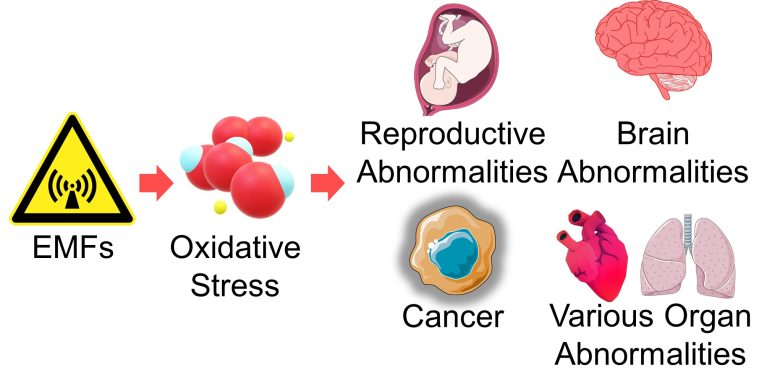
EMFs damage DNA and mitochondria by increasing ROS and also damage cells by triggering immune responses in the brain, etc.
This damage leads to mutations and cell death.
Mutations and cell death at the cellular level affect health, as they lead to diseases such as cancer, miscarriage, infertility, birth defects, neurodegenerative diseases, and developmental disorders.
Table of ContentsAll_Pages
Mutations and Cell Death from DNA Damage
EMFs produce ROS, which damage DNA, leading to mutations and cell death.
Playing a major role in this pathway is replication stress, a major factor in producing large-scale mutations such as chromosome structural abnormalities and aneuploidy.
Cells that have undergone large-scale mutations are induced to cell death, but not always.
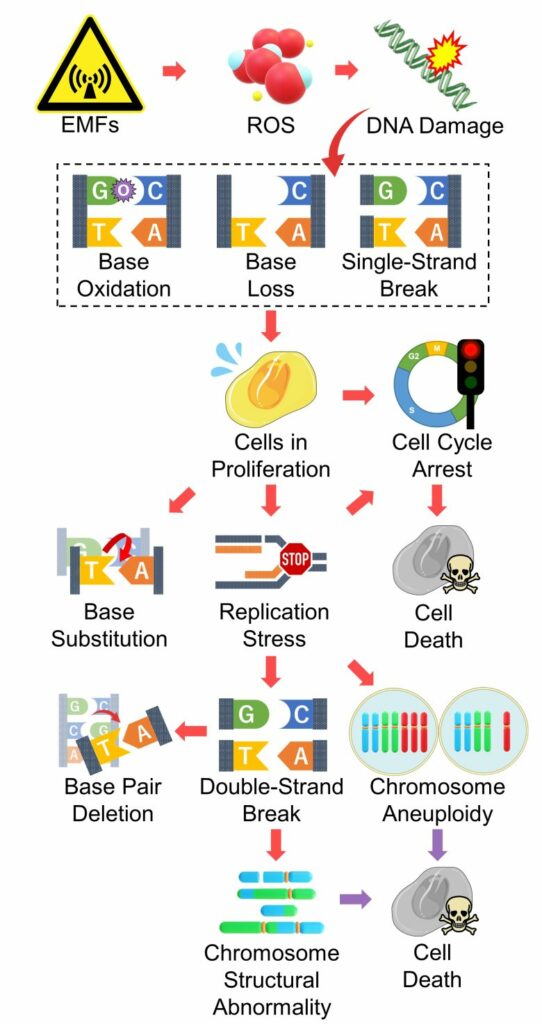
The point of this pathway is that EMFs damage DNA, and when this occurs in cells in proliferation, it leads to mutations and cell death.
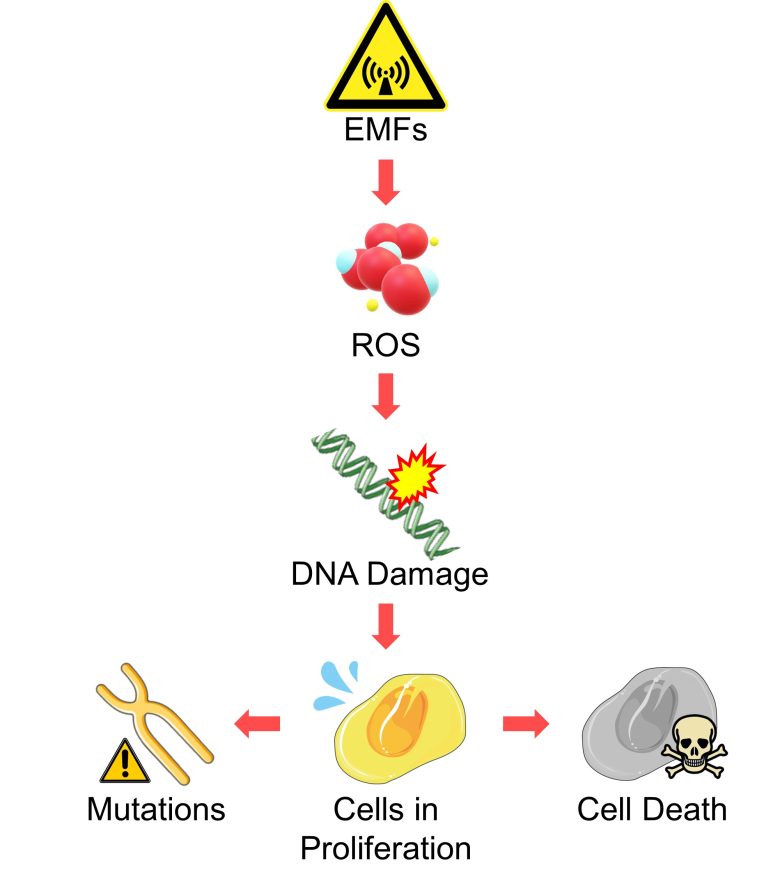
Therefore, it follows that younger people, who have more active cell division, are more vulnerable to EMFs, which is consistent with the findings of numerous epidemiological studies of EMFs.
Cell Death from Mitochondria Damage and Immune Responses
In addition, ROS produced by EMFs damage mitochondria and trigger immune responses in the brain, etc., which also induces cell death.
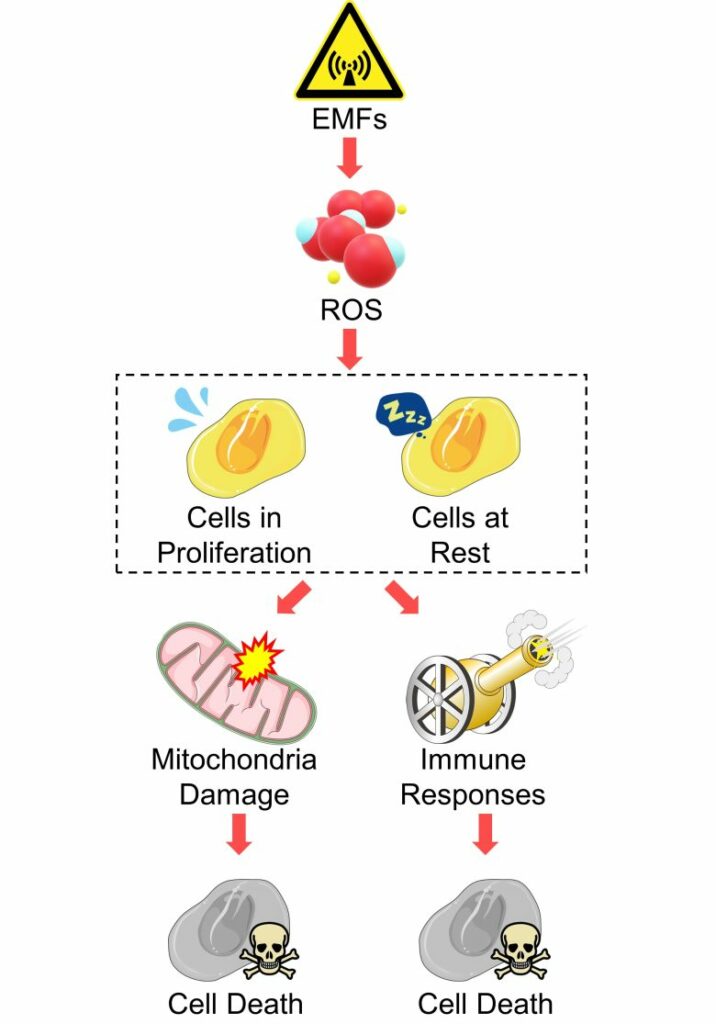
The point of this pathway is that it also applies to tissues that have little cell proliferation, such as the brain and heart.
Health Effects from Mutations
Cancer
When DNA is mutated, proteins produced from the DNA are denatured or their production is increased or decreased.
When this occurs, especially in tumor suppressor genes and proto-oncogenes, cell survival and proliferation is dysregulated, which can result in cancer.
In addition, cells that have developed chromosome structural abnormalities or aneuploidy can further exacerbate chromosome abnormalities during cell division, which can also result in cancer.
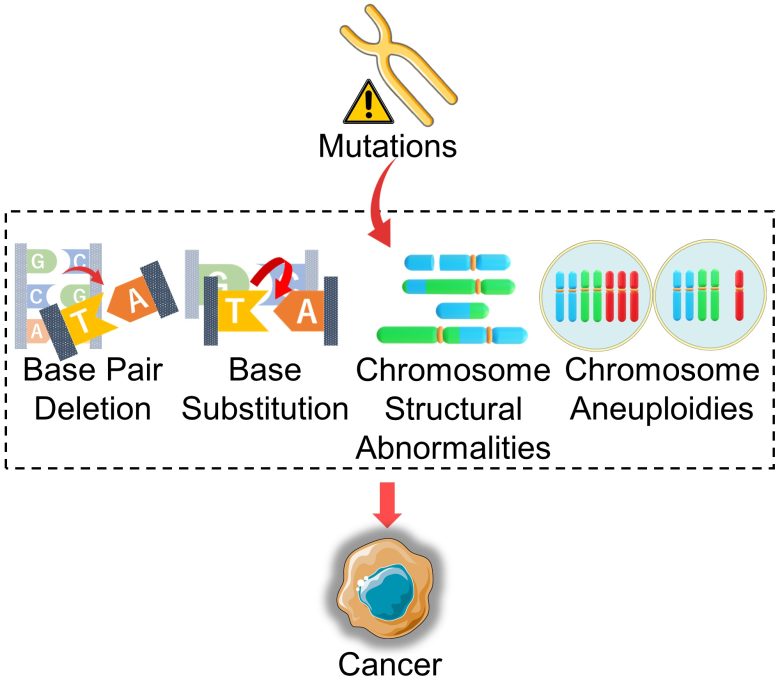
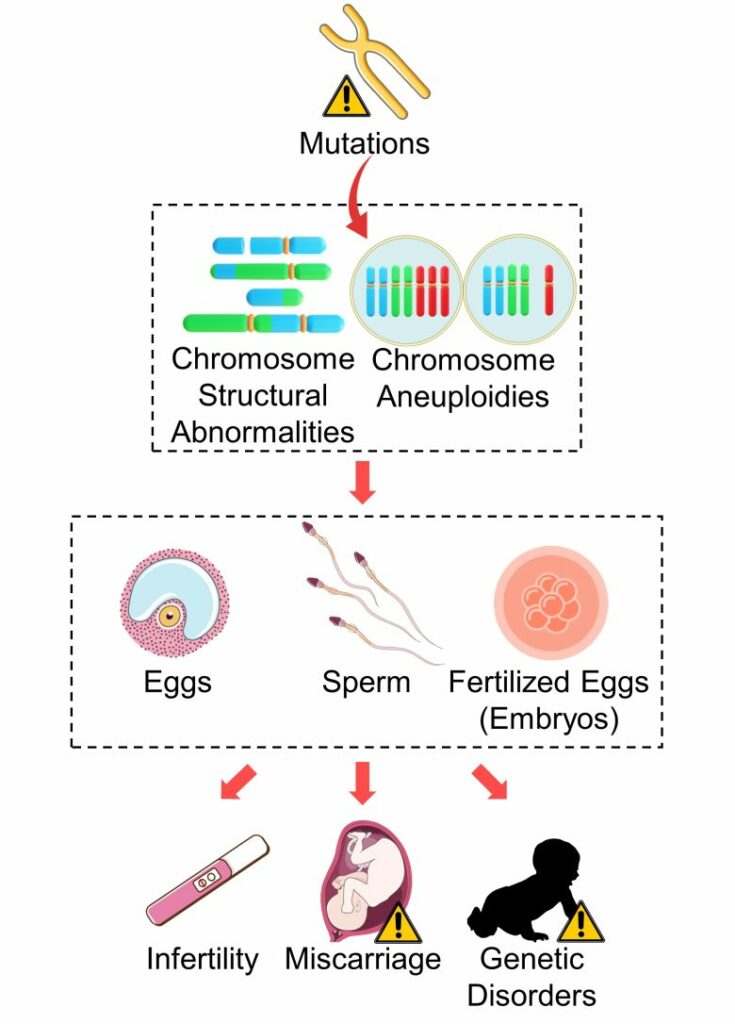
Infertility, Miscarriage, Genetic Disorders
When, among mutations, large-scale mutations such as chromosome structural abnormalities and aneuploidy occur in sperm, eggs, and fertilized eggs (embryos), it can lead to infertility, miscarriage, and genetic disorders.
Health Effects from Cell Death
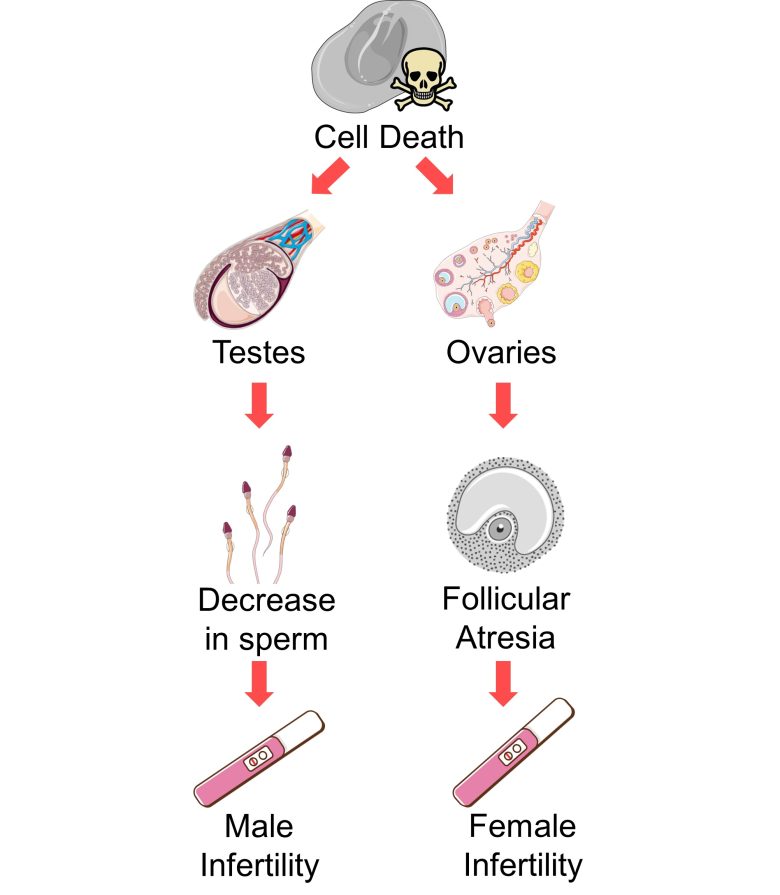
Infertility
An increase in cell death in the testes causes a decrease in sperm, which can lead to male infertility.
An increase in cell death in the ovaries causes an increase in follicular atresia, which can lead to female infertility.
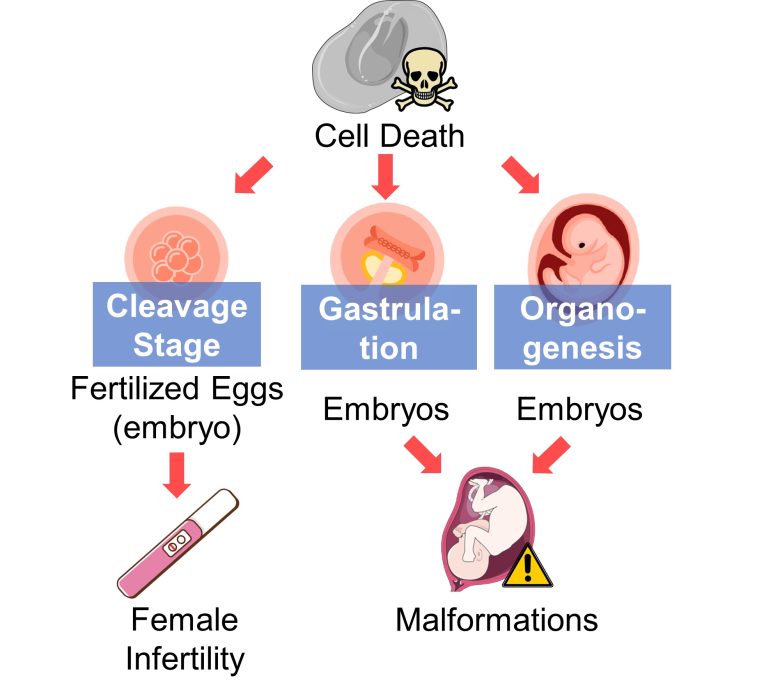
Miscarriage, Malformations
An increase in cell death in fertilized eggs (embryos) in the early stages, such as the cleavage stage, can lead to the loss of the embryos prior to implantation, i.e., female infertility.
An increase in cell death in embryos in the gastrulation and organogenesis can lead to malformations.
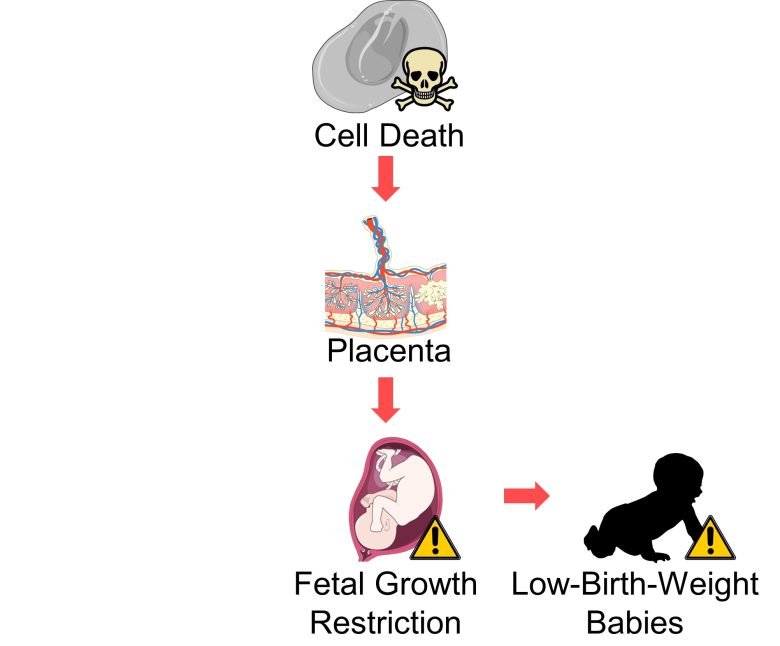
Low-Birth-Weight Babies
An increase in cell death in placental villi can lead to fetal growth restriction, resulting in low-birth-weight babies.
Although the evidence in the literature is weak, an increase in cell death throughout the fetal body, even if not limited to the placenta, may also lead to low-birth-weight babies
Neurodegenerative Disorders, Developmental Disorders, a Decline in Memory
An increase in cell death in the brain can lead to neurodegenerative diseases, developmental disorders, and a decline in memory.
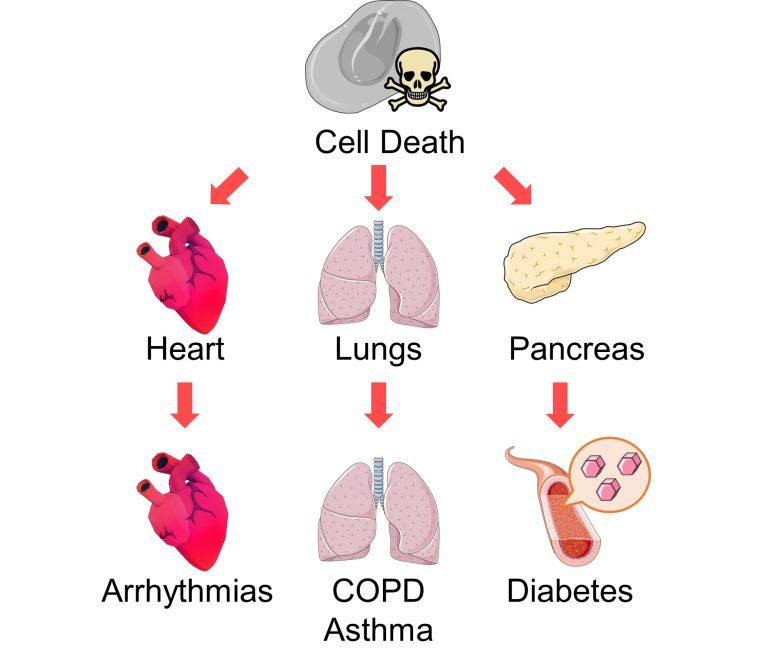
Various Organ Diseases
An increase in cell death in other organs, such as the heart, lungs, and pancreas, cab also lead to various diseases.
Health Effects from Cell Proliferation
Cancer Promotion
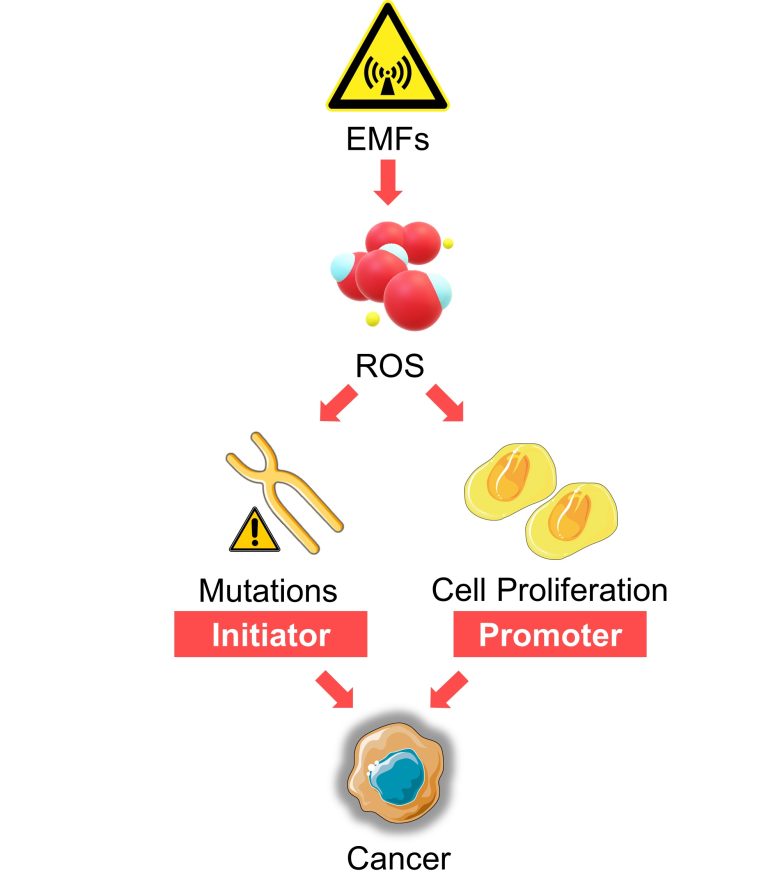
ROS produced by EMFs also function as signals for cell proliferation.
A possible health effect related to this is the promotion of cancer growth.
That is, EMFs are likely to function not only as an initiator of cancer by mutations, but also as a promoter of cancer by promoting cell proliferation.
It Backs Up EMF Health Effects
And numerous studies have shown that the diseases mentioned above are in fact increased by EMF exposure.
Therefore, the series of contents explained in this article backs up the health effects of EMFs in terms of mechanisms.
The studies showing the health effects of EMFs can be found below.
Numerous Studies Show EMF Health Effects

In this day and age, you might come off as a bit insane if you say that EMFs (Electric and Magnetic Fields) are bad for you. But this is in fact what numerous scientific papers are pointing to. I too was skeptical until I actually… Read the Full Article
Attribution of Images
The following images in this article are attributed to Servier Medical Art.
A cell, silhouette of the human body, tumor, blood vessel, glucose, kidney, lung, liver, fetus, DNA double strands, chromosome, sperm, follicle, DNA double strands with bases, cell with DNA, cell transition from chromosome aggregation to separation, bone, ovary, breast, intestine, stomach, differentiated cell, mitochondrion, large cell with cell membrane, Inflammatory cytokines, membrane protein, neural connection, glutamic acid, granular ATP, GABA, spermatogonium to sperm transition, testis, ovary, embryo transition during the cleavage stage, cell membrane, receptor, growth factor.
Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
References
1. Sengupta P. 2013. The Laboratory Rat: Relating Its Age With Human’s. International journal of preventive medicine. 4(6):624–30. [accessed 2024 Feb 6]. https://pubmed.ncbi.nlm.nih.gov/23930179/.
2. Dutta S, Sengupta P. 2016. Men and mice: Relating their ages. Life Sciences. 152:244–248. doi:10.1016/j.lfs.2015.10.025. http://dx.doi.org/10.1016/j.lfs.2015.10.025.
3. Amrhein V, Greenland S, McShane B. 2019. Scientists rise up against statistical significance. Nature. 567(7748):305–307. doi:10.1038/d41586-019-00857-9. http://dx.doi.org/10.1038/d41586-019-00857-9.
4. Fyodorov DV, Zhou B-R, Skoultchi AI, Bai Y. 2017. Emerging roles of linker histones in regulating chromatin structure and function. Nature Reviews Molecular Cell Biology. 19(3):192–206. doi:10.1038/nrm.2017.94. http://dx.doi.org/10.1038/nrm.2017.94.
5. Gyori BM, Venkatachalam G, Thiagarajan PS, Hsu D, Clement M-V. 2014. OpenComet: An automated tool for comet assay image analysis. Redox Biology. 2:457–465. doi:10.1016/j.redox.2013.12.020. http://dx.doi.org/10.1016/j.redox.2013.12.020.
6. Institute of Electrical and Electronics Engineers. Biological and health effects of electric and magnetic fields from video display terminals. [accessed 2023 Jul 6]. https://ieeexplore.ieee.org/document/585523.
7. The Electric Power Research Institute. Magnetic Fields from Electrical Appliances and Devices. [accessed 2023 Jul 18]. http://www.epri.com/research/products/000000000001021221.
8. Burhans WC, Heintz NH. 2009. The cell cycle is a redox cycle: Linking phase-specific targets to cell fate. Free Radical Biology and Medicine. 47(9):1282–1293. doi:10.1016/j.freeradbiomed.2009.05.026. http://dx.doi.org/10.1016/j.freeradbiomed.2009.05.026.
9. Rastogi RP, Richa, Kumar A, Tyagi MB, Sinha RP. 2010. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. Journal of Nucleic Acids. 2010:1–32. doi:10.4061/2010/592980. http://dx.doi.org/10.4061/2010/592980.
10. Boonstra J, Post JA. 2004. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 337:1–13. doi:10.1016/j.gene.2004.04.032. http://dx.doi.org/10.1016/j.gene.2004.04.032.
11. Koundrioukoff S, Carignon S, Técher H, Letessier A, Brison O, Debatisse M. 2013. Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity. Plon SE, editor. PLoS Genetics. 9(7):e1003643. doi:10.1371/journal.pgen.1003643. http://dx.doi.org/10.1371/journal.pgen.1003643.
12. Harris PA, Lamb J, Heaton B, Wheatley DN. 2002. Possible attenuation of the G2 DNA damage cell cycle checkpoint in HeLa cells by extremely low frequency (ELF) electromagnetic fields. Cancer cell international. 2(1):3. doi:10.1186/1475-2867-2-3. [accessed 2024 Feb 6]. https://pubmed.ncbi.nlm.nih.gov/12069691/.
13. Sancar A, Lindsey-Boltz LA, Kang T-H, Reardon JT, Lee JH, Ozturk N. 2010. Circadian clock control of the cellular response to DNA damage. FEBS Letters. 584(12):2618–2625. doi:10.1016/j.febslet.2010.03.017. http://dx.doi.org/10.1016/j.febslet.2010.03.017.
14. Khapre RV, Samsa WE, Kondratov RV. 2010. Circadian regulation of cell cycle: Molecular connections between aging and the circadian clock. Annals of Medicine. 42(6):404–415. doi:10.3109/07853890.2010.499134. http://dx.doi.org/10.3109/07853890.2010.499134.
15. Xing F, Zhan Q, He Y, Cui J, He S, Wang G. 2016. 1800MHz Microwave Induces p53 and p53-Mediated Caspase-3 Activation Leading to Cell Apoptosis In Vitro. Nakano H, editor. PLOS ONE. 11(9):e0163935. doi:10.1371/journal.pone.0163935. http://dx.doi.org/10.1371/journal.pone.0163935.
16. Huang C-Y, Chang C-W, Chen C-R, Chuang C-Y, Chiang C-S, Shu W-Y, Fan T-C, Hsu IC. 2014. Extremely Low-Frequency Electromagnetic Fields Cause G1 Phase Arrest through the Activation of the ATM-Chk2-p21 Pathway. Migliaccio A, editor. PLoS ONE. 9(8):e104732. doi:10.1371/journal.pone.0104732. http://dx.doi.org/10.1371/journal.pone.0104732.
17. Liu Y, Hong R, Yu Y, Weng E. 2003. Effects of extremely low frequency electromagnetic fields on apoptosis and cell cycle of mouse brain and liver cells. Zhonghua lao dong wei sheng zhi ye bing za zhi = Zhonghua laodong weisheng zhiyebing zazhi = Chinese journal of industrial hygiene and occupational diseases. 21(5):339–41. [accessed 2024 Feb 6]. https://pubmed.ncbi.nlm.nih.gov/14761394/.
18. Kesari KK, Kumar S, Behari J. 2011. Effects of Radiofrequency Electromagnetic Wave Exposure from Cellular Phones on the Reproductive Pattern in Male Wistar Rats. Applied Biochemistry and Biotechnology. 164(4):546–559. doi:10.1007/s12010-010-9156-0. http://dx.doi.org/10.1007/s12010-010-9156-0.
19. Salem MA. 2008. Protective effect of Vitamin A against skin injury caused by exposure to electromagnetic field. The Egyptian Journal of Hospital Medicine. 32(1):352–366. doi:10.21608/ejhm.2008.18961. http://dx.doi.org/10.21608/ejhm.2008.18961.
20. Pandey N, Giri S, Das S, Upadhaya P. 2016. Radiofrequency radiation (900 MHz)-induced DNA damage and cell cycle arrest in testicular germ cells in swiss albino mice. Toxicology and Industrial Health. 33(4):373–384. doi:10.1177/0748233716671206. http://dx.doi.org/10.1177/0748233716671206.
21. Bin-Meferij MM, El-Kott AF. 2015. The radioprotective effects of Moringa oleifera against mobile phone electromagnetic radiation-induced infertility in rats. International journal of clinical and experimental medicine. 8(8):12487–97. [accessed 2024 Feb 6]. https://pubmed.ncbi.nlm.nih.gov/26550159/.
22. Harris PA, Lamb J, Heaton B, Wheatley DN. 2002. Cancer Cell International. 2(1):3. doi:10.1186/1475-2867-2-3. http://dx.doi.org/10.1186/1475-2867-2-3.
23. Zorov DB, Juhaszova M, Sollott SJ. 2006. Mitochondrial ROS-induced ROS release: An update and review. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1757(5–6):509–517. doi:10.1016/j.bbabio.2006.04.029. http://dx.doi.org/10.1016/j.bbabio.2006.04.029.
24. Batandier C, Leverve X, Fontaine E. 2004. Opening of the Mitochondrial Permeability Transition Pore Induces Reactive Oxygen Species Production at the Level of the Respiratory Chain Complex I. Journal of Biological Chemistry. 279(17):17197–17204. doi:10.1074/jbc.m310329200. http://dx.doi.org/10.1074/jbc.m310329200.
25. Zorov DB, Juhaszova M, Sollott SJ. 2014. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiological Reviews. 94(3):909–950. doi:10.1152/physrev.00026.2013. http://dx.doi.org/10.1152/physrev.00026.2013.
26. Halestrap AP, Doran E, Gillespie JP, O’Toole A. 2000. Mitochondria and cell death. Biochemical Society Transactions. 28(2):170–177. doi:10.1042/bst0280170. http://dx.doi.org/10.1042/bst0280170.
27. Halestrap AP. 2009. What is the mitochondrial permeability transition pore? Journal of Molecular and Cellular Cardiology. 46(6):821–831. doi:10.1016/j.yjmcc.2009.02.021. http://dx.doi.org/10.1016/j.yjmcc.2009.02.021.
28. Zuo H, Lin T, Wang D, Peng R, Wang Shuiming, Gao Y, Xu X, Li Y, Wang Shaoxia, Zhao L, et al. 2014. Neural Cell Apoptosis Induced by Microwave Exposure Through Mitochondria-dependent Caspase-3 Pathway. International Journal of Medical Sciences. 11(5):426–435. doi:10.7150/ijms.6540. http://dx.doi.org/10.7150/ijms.6540.
29. Liu Yu-xiao, Tai J, Li G, Zhang Z, Xue J, Liu H, Zhu H, Cheng J, Liu Yuan-ling, Li A, et al. 2012. Exposure to 1950-MHz TD-SCDMA Electromagnetic Fields Affects the Apoptosis of Astrocytes via Caspase-3-Dependent Pathway. Castresana JS, editor. PLoS ONE. 7(8):e42332. doi:10.1371/journal.pone.0042332. http://dx.doi.org/10.1371/journal.pone.0042332.
30. Kiray A, Tayefi H, Kiray M, Bagriyanik HA, Pekcetin C, Ergur BU, Ozogul C. 2012. The effects of exposure to electromagnetic field on rat myocardium. Toxicology and Industrial Health. 29(5):418–425. doi:10.1177/0748233711434957. http://dx.doi.org/10.1177/0748233711434957.
31. Türedi S, Hancı H, Topal Z, Ünal D, Mercantepe T, Bozkurt İ, Kaya H, Odacı E. 2014. The effects of prenatal exposure to a 900-MHz electromagnetic field on the 21-day-old male rat heart. Electromagnetic Biology and Medicine. 34(4):390–397. doi:10.3109/15368378.2014.952742. http://dx.doi.org/10.3109/15368378.2014.952742.
32. Liu YQ, Gao YB, Dong J, Yao BW, Zhao L, Peng RY. 2015. Pathological changes in the sinoatrial node tissues of rats caused by pulsed microwave exposure. Biomedical and environmental sciences : BES. 28(1):72–5. doi:10.3967/bes2015.007. [accessed 2024 Feb 6]. https://pubmed.ncbi.nlm.nih.gov/25566864/.
33. Giacalone M, Di Sacco F, Traupe I, Pagnucci N, Forfori F, Giunta F. 2015. Blueberry Polyphenols and Neuroprotection. Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease.:17–28. doi:10.1016/b978-0-12-411462-3.00002-3. http://dx.doi.org/10.1016/b978-0-12-411462-3.00002-3.
34. Heneka MT, Kummer MP, Latz E. 2014. Innate immune activation in neurodegenerative disease. Nature Reviews Immunology. 14(7):463–477. doi:10.1038/nri3705. http://dx.doi.org/10.1038/nri3705.
35. Ben Haim L, Carrillo-de Sauvage M-A, Ceyzériat K, Escartin C. 2015. Elusive roles for reactive astrocytes in neurodegenerative diseases. Frontiers in Cellular Neuroscience. 9. doi:10.3389/fncel.2015.00278. http://dx.doi.org/10.3389/fncel.2015.00278.
36. Chatterjee S. 2016. Oxidative Stress, Inflammation, and Disease. Oxidative Stress and Biomaterials.:35–58. doi:10.1016/b978-0-12-803269-5.00002-4. http://dx.doi.org/10.1016/b978-0-12-803269-5.00002-4.
37. Hayden MS, West AP, Ghosh S. 2006. NF-κB and the immune response. Oncogene. 25(51):6758–6780. doi:10.1038/sj.onc.1209943. http://dx.doi.org/10.1038/sj.onc.1209943.
38. Gloire G, Legrand-Poels S, Piette J. 2006. NF-κB activation by reactive oxygen species: Fifteen years later. Biochemical Pharmacology. 72(11):1493–1505. doi:10.1016/j.bcp.2006.04.011. http://dx.doi.org/10.1016/j.bcp.2006.04.011.
39. Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. 2019. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 224(2):242–253. doi:10.1016/j.imbio.2018.11.010. http://dx.doi.org/10.1016/j.imbio.2018.11.010.
40. Bordt EA, Polster BM. 2014. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: a bipartisan affair? Free Radical Biology and Medicine. 76:34–46. doi:10.1016/j.freeradbiomed.2014.07.033. http://dx.doi.org/10.1016/j.freeradbiomed.2014.07.033.
41. Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, et al. 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 541(7638):481–487. doi:10.1038/nature21029. http://dx.doi.org/10.1038/nature21029.
42. van Hall G, Stømstad M, Rasmussen P, Jans Ø, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB. 2009. Blood Lactate is an Important Energy Source for the Human Brain. Journal of Cerebral Blood Flow & Metabolism. 29(6):1121–1129. doi:10.1038/jcbfm.2009.35. http://dx.doi.org/10.1038/jcbfm.2009.35.
43. Salińska E, Danysz W, Łazarewicz JW. 2005. The role of excitotoxicity in neurodegeneration. Folia neuropathologica. 43(4):322–39. [accessed 2024 Feb 6]. https://pubmed.ncbi.nlm.nih.gov/16416396/.
44. Scemes E, Giaume C. 2006. Astrocyte calcium waves: What they are and what they do. Glia. 54(7):716–725. doi:10.1002/glia.20374. http://dx.doi.org/10.1002/glia.20374.
45. Butt AM. 2011. ATP: A ubiquitous gliotransmitter integrating neuron–glial networks. Seminars in Cell & Developmental Biology. 22(2):205–213. doi:10.1016/j.semcdb.2011.02.023. http://dx.doi.org/10.1016/j.semcdb.2011.02.023.
46. Ding S, Fellin T, Zhu Y, Lee S-Y, Auberson YP, Meaney DF, Coulter DA, Carmignoto G, Haydon PG. 2007. Enhanced Astrocytic Ca2+Signals Contribute to Neuronal Excitotoxicity after Status Epilepticus. The Journal of Neuroscience. 27(40):10674–10684. doi:10.1523/jneurosci.2001-07.2007. http://dx.doi.org/10.1523/jneurosci.2001-07.2007.
47. Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, et al. 2014. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nature Medicine. 20(8):886–896. doi:10.1038/nm.3639. http://dx.doi.org/10.1038/nm.3639.
48. Wu Z, Guo Z, Gearing M, Chen G. 2014. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s disease model. Nature Communications. 5(1). doi:10.1038/ncomms5159. http://dx.doi.org/10.1038/ncomms5159.
49. Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. 2005. Synaptic Activity Regulates Interstitial Fluid Amyloid-β Levels In Vivo. Neuron. 48(6):913–922. doi:10.1016/j.neuron.2005.10.028. http://dx.doi.org/10.1016/j.neuron.2005.10.028.
50. Zhao J, O’Connor T, Vassar R. 2011. The contribution of activated astrocytes to Aβ production: Implications for Alzheimer’s disease pathogenesis. Journal of Neuroinflammation. 8(1). doi:10.1186/1742-2094-8-150. http://dx.doi.org/10.1186/1742-2094-8-150.
51. Hu J, Van Eldik LJ. 1999. Glial-derived proteins activate cultured astrocytes and enhance beta amyloid-induced glial activation. Brain Research. 842(1):46–54. doi:10.1016/s0006-8993(99)01804-1. http://dx.doi.org/10.1016/s0006-8993(99)01804-1.
52. Brown GC. 2007. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochemical Society Transactions. 35(5):1119–1121. doi:10.1042/bst0351119. http://dx.doi.org/10.1042/bst0351119.
53. Ascenzi P, di Masi A, Sciorati C, Clementi E. 2010. Peroxynitrite—An ugly biofactor? BioFactors. 36(4):264–273. doi:10.1002/biof.103. http://dx.doi.org/10.1002/biof.103.
54. Yang X, He G, Hao Y, Chen C, Li M, Wang Y, Zhang G, Yu Z. 2010. The role of the JAK2-STAT3 pathway in pro-inflammatory responses of EMF-stimulated N9 microglial cells. Journal of Neuroinflammation. 7(1). doi:10.1186/1742-2094-7-54. http://dx.doi.org/10.1186/1742-2094-7-54.
55. Megha K, Deshmukh PS, Banerjee BD, Tripathi AK, Ahmed R, Abegaonkar MP. 2015. Low intensity microwave radiation induced oxidative stress, inflammatory response and DNA damage in rat brain. NeuroToxicology. 51:158–165. doi:10.1016/j.neuro.2015.10.009. http://dx.doi.org/10.1016/j.neuro.2015.10.009.
56. MAUSSETBONNEFONT A, HIRBEC H, BONNEFONT X, PRIVAT A, VIGNON J, DESEZE R. 2004. Acute exposure to GSM 900-MHz electromagnetic fields induces glial reactivity and biochemical modifications in the rat brain. Neurobiology of Disease. 17(3):445–454. doi:10.1016/j.nbd.2004.07.004. http://dx.doi.org/10.1016/j.nbd.2004.07.004.
57. Ammari M, Gamez C, Lecomte A, Sakly M, Abdelmelek H, De Seze R. 2010. GFAP expression in the rat brain following sub-chronic exposure to a 900 MHz electromagnetic field signal. International Journal of Radiation Biology. 86(5):367–375. doi:10.3109/09553000903567946. http://dx.doi.org/10.3109/09553000903567946.
58. Akakin D, Tok OE, Anil D, Akakin A, Sirvanci S, Sener G, Ercan F. 2020. Electromagnetic waves from mobile phones may affect rat brain during development. Turkish Neurosurgery. doi:10.5137/1019-5149.jtn.31665-20.2. http://dx.doi.org/10.5137/1019-5149.jtn.31665-20.2.
59. Afeefy AA, Afifi OK, Tolba AMA. 2013. A Histological and Immunohistochemical study on the effect of mobile phone radiation on the hipocampus of adult and newborn albino rats. Nature and Science. 11(8):98–113. https://www.researchgate.net/publication/320922211_A_Histological_and_Immunohistochemical_study_on_the_effect_of_mobile_phone_radiation_on_the_hipocampus_of_adult_and_newborn_albino_rats.
60. Scaltriti M, Baselga J. 2006. The Epidermal Growth Factor Receptor Pathway: A Model for Targeted Therapy. Clinical Cancer Research. 12(18):5268–5272. doi:10.1158/1078-0432.ccr-05-1554. http://dx.doi.org/10.1158/1078-0432.ccr-05-1554.
61. Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. 2005. Intracellular Reactive Oxygen Species Activate Src Tyrosine Kinase during Cell Adhesion and Anchorage-Dependent Cell Growth. Molecular and Cellular Biology. 25(15):6391–6403. doi:10.1128/mcb.25.15.6391-6403.2005. http://dx.doi.org/10.1128/mcb.25.15.6391-6403.2005.
62. Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiroi Y, Yazaki Y. 1997. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. Journal of Clinical Investigation. 100(7):1813–1821. doi:10.1172/jci119709. http://dx.doi.org/10.1172/jci119709.
63. Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. 1998. Negative Regulation of PKB/Akt-Dependent Cell Survival by the Tumor Suppressor PTEN. Cell. 95(1):29–39. doi:10.1016/s0092-8674(00)81780-8. http://dx.doi.org/10.1016/s0092-8674(00)81780-8.
64. Wu KLH, Wu C-A, Wu C-W, Chan SHH, Chang AYW, Chan JYH. 2013. Redox-Sensitive Oxidation and Phosphorylation of PTEN Contribute to Enhanced Activation of PI3K/Akt Signaling in Rostral Ventrolateral Medulla and Neurogenic Hypertension in Spontaneously Hypertensive Rats. Antioxidants & Redox Signaling. 18(1):36–50. doi:10.1089/ars.2011.4457. http://dx.doi.org/10.1089/ars.2011.4457.
65. Ciccone M, Calin GA, Perrotti D. 2015. From the Biology of PP2A to the PADs for Therapy of Hematologic Malignancies. Frontiers in Oncology. 5. doi:10.3389/fonc.2015.00021. http://dx.doi.org/10.3389/fonc.2015.00021.
66. Nakahata S, Morishita K. 2014. PP2A inactivation by ROS accumulation. Blood. 124(14):2163–2165. doi:10.1182/blood-2014-08-594093. http://dx.doi.org/10.1182/blood-2014-08-594093.
67. Shimura T, Sasatani M, Kamiya K, Kawai H, Inaba Y, Kunugita N. 2015. Mitochondrial reactive oxygen species perturb AKT/cyclin D1 cell cycle signaling via oxidative inactivation of PP2A in lowdose irradiated human fibroblasts. Oncotarget. 7(3):3559–3570. doi:10.18632/oncotarget.6518. http://dx.doi.org/10.18632/oncotarget.6518.
68. Chiarugi P, Cirri P. 2003. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends in Biochemical Sciences. 28(9):509–514. doi:10.1016/s0968-0004(03)00174-9. http://dx.doi.org/10.1016/s0968-0004(03)00174-9.
69. Meves A, Stock SN, Beyerle A, Pittelkow MR, Peus D. 2001. H2O2 mediates oxidative stress-induced epidermal growth factor receptor phosphorylation. Toxicology Letters. 122(3):205–214. doi:10.1016/s0378-4274(01)00359-9. http://dx.doi.org/10.1016/s0378-4274(01)00359-9.
70. Martínez MA, Úbeda A, Cid MA, Trillo MÁ. 2012. The Proliferative Response of NB69 Human Neuroblastoma Cells to a 50 Hz Magnetic Field is mediated by ERK1/2 Signaling. Cellular Physiology and Biochemistry. 29(5–6):675–686. doi:10.1159/000178457. http://dx.doi.org/10.1159/000178457.
71. Velizarov S, Raskmark P, Kwee S. 1999. The effects of radiofrequency fields on cell proliferation are non-thermal. Bioelectrochemistry and Bioenergetics. 48(1):177–180. doi:10.1016/s0302-4598(98)00238-4. http://dx.doi.org/10.1016/s0302-4598(98)00238-4.
72. Tang Q, Zhao N. 1999. Effects of low frequency electromagnetic fields on osteoblasts proliferation and cell cycle. Chinese Science Bulletin. 44(23):2174–2177. doi:10.1007/bf03182703. http://dx.doi.org/10.1007/bf03182703.
73. Zhong C, Zhang X, Xu Z, He R. 2012. Effects of Low-Intensity Electromagnetic Fields on the Proliferation and Differentiation of Cultured Mouse Bone Marrow Stromal Cells. Physical Therapy. 92(9):1208–1219. doi:10.2522/ptj.20110224. http://dx.doi.org/10.2522/ptj.20110224.
74. Phillips JL, Rutledge L, Winters WD. 1986. Transferrin binding to two human colon carcinoma cell lines: characterization and effect of 60-Hz electromagnetic fields. Cancer research. 46(1):239–44. [accessed 2024 Feb 6]. https://pubmed.ncbi.nlm.nih.gov/2998606/.
75. Kumar N, Singh AK. 2015. Trends of male factor infertility, an important cause of infertility: A review of literature. Journal of human reproductive sciences. 8(4):191–6. doi:10.4103/0974-1208.170370. [accessed 2024 Feb 6]. https://pubmed.ncbi.nlm.nih.gov/26752853/.
76. Stringer JM, Winship A, Zerafa N, Wakefield M, Hutt K. 2020. Oocytes can efficiently repair DNA double-strand breaks to restore genetic integrity and protect offspring health. Proceedings of the National Academy of Sciences. 117(21):11513–11522. doi:10.1073/pnas.2001124117. http://dx.doi.org/10.1073/pnas.2001124117.
77. SUGINO N. 2005. Reactive oxygen species in ovarian physiology. Reproductive Medicine and Biology. 4(1):31–44. doi:10.1111/j.1447-0578.2005.00086.x. http://dx.doi.org/10.1111/j.1447-0578.2005.00086.x.
78. Yao W, Pan Z, Du X, Zhang J, Liu H, Li Q. 2021. NORHA, a novel follicular atresia-related lncRNA, promotes porcine granulosa cell apoptosis via the miR-183-96-182 cluster and FoxO1 axis. Journal of Animal Science and Biotechnology. 12(1). doi:10.1186/s40104-021-00626-7. http://dx.doi.org/10.1186/s40104-021-00626-7.
79. İdil M, Çepni İ, Demirsoy G, Öcal P, Salihoğlu F, Şenol H, Elibol F, İrez T. 2004. Does granulosa cell apoptosis have a role in the etiology of unexplained infertility? European Journal of Obstetrics & Gynecology and Reproductive Biology. 112(2):182–184. doi:10.1016/s0301-2115(03)00365-8. http://dx.doi.org/10.1016/s0301-2115(03)00365-8.
80. Mohebi M, Ghafouri-Fard S. 2019. Embryo developmental arrest: Review of genetic factors and pathways. Gene Reports. 17:100479. doi:10.1016/j.genrep.2019.100479. http://dx.doi.org/10.1016/j.genrep.2019.100479.
81. Hardy K. 1999. Apoptosis in the human embryo. Reviews of Reproduction. 4(3):125–134. doi:10.1530/ror.0.0040125. http://dx.doi.org/10.1530/ror.0.0040125.
82. Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. 2000. Blastocyst quality affects the success of blastocyst-stage embryo transfer. Fertility and Sterility. 74(2):282–287. doi:10.1016/s0015-0282(00)00645-2. http://dx.doi.org/10.1016/s0015-0282(00)00645-2.
83. Seshagiri PB, Sen Roy S, Sireesha G, Rao RP. 2009. Cellular and molecular regulation of mammalian blastocyst hatching. Journal of Reproductive Immunology. 83(1–2):79–84. doi:10.1016/j.jri.2009.06.264. http://dx.doi.org/10.1016/j.jri.2009.06.264.
84. Sulik KK, Cook CS, Webster WS. 1988. Teratogens and craniofacial malformations: relationships to cell death. Development. 103(Supplement):213–232. doi:10.1242/dev.103.supplement.213. http://dx.doi.org/10.1242/dev.103.supplement.213.
85. Knobloch J, Rüther U. 2008. Shedding light on an old mystery: Thalidomide suppresses survival pathways to induce limb defects. Cell Cycle. 7(9):1121–1127. doi:10.4161/cc.7.9.5793. http://dx.doi.org/10.4161/cc.7.9.5793.
86. Zhao Z, Reece EA. 2005. Nicotine-induced embryonic malformations mediated by apoptosis from increasing intracellular calcium and oxidative stress. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 74(5):383–391. doi:10.1002/bdrb.20052. http://dx.doi.org/10.1002/bdrb.20052.
87. Scifres CM, Nelson DM. 2009. Intrauterine growth restriction, human placental development and trophoblast cell death. The Journal of Physiology. 587(14):3453–3458. doi:10.1113/jphysiol.2009.173252. http://dx.doi.org/10.1113/jphysiol.2009.173252.
88. Ishii T, Miyazawa M, Onodera A, Yasuda K, Kawabe N, Kirinashizawa M, Yoshimura S, Maruyama N, Hartman PS, Ishii N. 2011. Mitochondrial reactive oxygen species generation by the SDHC V69E mutation causes low birth weight and neonatal growth retardation. Mitochondrion. 11(1):155–165. doi:10.1016/j.mito.2010.09.006. http://dx.doi.org/10.1016/j.mito.2010.09.006.
89. Phatnani H, Maniatis T. 2015. Astrocytes in Neurodegenerative Disease: Table 1. Cold Spring Harbor Perspectives in Biology. 7(6):a020628. doi:10.1101/cshperspect.a020628. http://dx.doi.org/10.1101/cshperspect.a020628.
90. Smith JA, Das A, Ray SK, Banik NL. 2012. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Research Bulletin. 87(1):10–20. doi:10.1016/j.brainresbull.2011.10.004. http://dx.doi.org/10.1016/j.brainresbull.2011.10.004.
91. Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. 2012. Mitochondria, oxidative stress and neurodegeneration. Journal of the Neurological Sciences. 322(1–2):254–262. doi:10.1016/j.jns.2012.05.030. http://dx.doi.org/10.1016/j.jns.2012.05.030.
92. Wang X, Wang W, Li L, Perry G, Lee H, Zhu X. 2014. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1842(8):1240–1247. doi:10.1016/j.bbadis.2013.10.015. http://dx.doi.org/10.1016/j.bbadis.2013.10.015.
93. Liu X, Xu S, Wang P, Wang W. 2015. Transient mitochondrial permeability transition mediates excitotoxicity in glutamate-sensitive NSC34 D motor neuron-like cells. Experimental Neurology. 271:122–130. doi:10.1016/j.expneurol.2015.05.010. http://dx.doi.org/10.1016/j.expneurol.2015.05.010.
94. Choi I-Y, Lim J-H, Kim C, Song HY, Ju C, Kim W-K. 2013. 4-hydroxy-2(E)-Nonenal facilitates NMDA-Induced Neurotoxicity via Triggering Mitochondrial Permeability Transition Pore Opening and Mitochondrial Calcium Overload. Experimental Neurobiology. 22(3):200–207. doi:10.5607/en.2013.22.3.200. http://dx.doi.org/10.5607/en.2013.22.3.200.
95. Dhikav V, Anand K. 2011. Potential Predictors of Hippocampal Atrophy in Alzheimerʼs Disease. Drugs & Aging. 28(1):1–11. doi:10.2165/11586390-000000000-00000. http://dx.doi.org/10.2165/11586390-000000000-00000.
96. Jahn H. 2013. Memory loss in Alzheimer’s disease. Dialogues in Clinical Neuroscience. 15(4):445–454. doi:10.31887/dcns.2013.15.4/hjahn. http://dx.doi.org/10.31887/dcns.2013.15.4/hjahn.
97. Videbech P. 2004. Hippocampal Volume and Depression: A Meta-Analysis of MRI Studies. American Journal of Psychiatry. 161(11):1957–1966. doi:10.1176/appi.ajp.161.11.1957. http://dx.doi.org/10.1176/appi.ajp.161.11.1957.
98. Burt DB, Zembar MJ, Niederehe G. 1995. Depression and memory impairment: A meta-analysis of the association, its pattern, and specificity. Psychological Bulletin. 117(2):285–305. doi:10.1037/0033-2909.117.2.285. http://dx.doi.org/10.1037/0033-2909.117.2.285.
99. Isaacs EB, Lucas A, Chong WK, Wood SJ, Johnson CL, Marshall C, Vargha-Khadem F, Gadian DG. 2000. Hippocampal Volume and Everyday Memory in Children of Very Low Birth Weight. Pediatric Research. 47(6):713–720. doi:10.1203/00006450-200006000-00006. http://dx.doi.org/10.1203/00006450-200006000-00006.
100. Aanes S, Bjuland KJ, Sripada K, Sølsnes AE, Grunewaldt KH, Håberg A, Løhaugen GC, Skranes J. 2019. Reduced hippocampal subfield volumes and memory function in school-aged children born preterm with very low birthweight (VLBW). NeuroImage: Clinical. 23:101857. doi:10.1016/j.nicl.2019.101857. http://dx.doi.org/10.1016/j.nicl.2019.101857.
101. Bigler ED, Johnson SC, Anderson CV, Blatter DD, Gale SD, Russo AA, Ryser DK, Macnamara SE, Bailey BJ, Hopkins RO, et al. 1996. Traumatic brain injury and memory: The role of hippocampal atrophy. Neuropsychology. 10(3):333–342. doi:10.1037/0894-4105.10.3.333. http://dx.doi.org/10.1037/0894-4105.10.3.333.
102. Monti JM, Voss MW, Pence A, McAuley E, Kramer AF, Cohen NJ. 2013. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Frontiers in Aging Neuroscience. 5. doi:10.3389/fnagi.2013.00041. http://dx.doi.org/10.3389/fnagi.2013.00041.
103. Krain AL, Castellanos FX. 2006. Brain development and ADHD. Clinical Psychology Review. 26(4):433–444. doi:10.1016/j.cpr.2006.01.005. http://dx.doi.org/10.1016/j.cpr.2006.01.005.
104. Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, van Hulzen KJE, Medland SE, Shumskaya E, Jahanshad N, et al. 2017. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. The Lancet Psychiatry. 4(4):310–319. doi:10.1016/s2215-0366(17)30049-4. http://dx.doi.org/10.1016/s2215-0366(17)30049-4.
105. Kinnear Kern J. 2003. Purkinje cell vulnerability and autism: a possible etiological connection. Brain and Development. 25(6):377–382. doi:10.1016/s0387-7604(03)00056-1. http://dx.doi.org/10.1016/s0387-7604(03)00056-1.
106. Piek A, de Boer RA, Silljé HHW. 2016. The fibrosis-cell death axis in heart failure. Heart Failure Reviews. 21(2):199–211. doi:10.1007/s10741-016-9536-9. http://dx.doi.org/10.1007/s10741-016-9536-9.
107. Piek A, de Boer RA, Silljé HHW. 2016. The fibrosis-cell death axis in heart failure. Heart Failure Reviews. 21(2):199–211. doi:10.1007/s10741-016-9536-9. http://dx.doi.org/10.1007/s10741-016-9536-9.
108. Kazbanov IV, ten Tusscher KHWJ, Panfilov AV. 2016. Effects of Heterogeneous Diffuse Fibrosis on Arrhythmia Dynamics and Mechanism. Scientific Reports. 6(1). doi:10.1038/srep20835. http://dx.doi.org/10.1038/srep20835.
109. Verheule S, Schotten U. 2021. Electrophysiological Consequences of Cardiac Fibrosis. Cells. 10(11):3220. doi:10.3390/cells10113220. http://dx.doi.org/10.3390/cells10113220.
110. Morita N, Mandel WJ, Kobayashi Y, Karagueuzian HS. 2014. Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. Journal of Arrhythmia. 30(6):389–394. doi:10.1016/j.joa.2013.12.008. http://dx.doi.org/10.1016/j.joa.2013.12.008.
111. Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. 2006. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respiratory Research. 7(1). doi:10.1186/1465-9921-7-53. http://dx.doi.org/10.1186/1465-9921-7-53.
112. Hamid Q, Tulic M. 2009. Immunobiology of Asthma. Annual Review of Physiology. 71(1):489–507. doi:10.1146/annurev.physiol.010908.163200. http://dx.doi.org/10.1146/annurev.physiol.010908.163200.
113. Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O. 2011. Oxidative Stress in Asthma. World Allergy Organization Journal. 4(10):151–158. doi:10.1097/wox.0b013e318232389e. http://dx.doi.org/10.1097/wox.0b013e318232389e.
114. Bush A. 2008. COPD: A Pediatric Disease. COPD: Journal of Chronic Obstructive Pulmonary Disease. 5(1):53–67. doi:10.1080/15412550701815965. http://dx.doi.org/10.1080/15412550701815965.
115. Gibson PG, Simpson JL. 2009. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 64(8):728–735. doi:10.1136/thx.2008.108027. http://dx.doi.org/10.1136/thx.2008.108027.
116. Bisgaard H, Jensen SM, Bønnelykke K. 2012. Interaction between Asthma and Lung Function Growth in Early Life. American Journal of Respiratory and Critical Care Medicine. 185(11):1183–1189. doi:10.1164/rccm.201110-1922oc. http://dx.doi.org/10.1164/rccm.201110-1922oc.
117. Bucchieri F, Puddicombe SM, Lordan JL, Richter A, Buchanan D, Wilson SJ, Ward J, Zummo G, Howarth PH, Djukanović R, et al. 2002. Asthmatic Bronchial Epithelium Is More Susceptible to Oxidant-Induced Apoptosis. American Journal of Respiratory Cell and Molecular Biology. 27(2):179–185. doi:10.1165/ajrcmb.27.2.4699. http://dx.doi.org/10.1165/ajrcmb.27.2.4699.
118. Schwartz J. 1997. Selective elimination of human lymphoid cells with unstable chromosome aberrations by p53-dependent apoptosis. Carcinogenesis. 18(1):201–205. doi:10.1093/carcin/18.1.201. http://dx.doi.org/10.1093/carcin/18.1.201.
119. Belloni P, Meschini R, Lewinska D, Palitti F. 2008. Apoptosis Preferentially Eliminates Irradiated G0Human Lymphocytes Bearing Dicentric Chromosomes. Radiation Research. 169(2):181–187. doi:10.1667/rr1158.1. http://dx.doi.org/10.1667/rr1158.1.
120. Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. 1999. p53- and ATM-Dependent Apoptosis Induced by Telomeres Lacking TRF2. Science. 283(5406):1321–1325. doi:10.1126/science.283.5406.1321. http://dx.doi.org/10.1126/science.283.5406.1321.
121. Lips J, Kaina B. 2001. DNA double-strand breaks trigger apoptosis in p53-deficient fibroblasts. Carcinogenesis. 22(4):579–585. doi:10.1093/carcin/22.4.579. http://dx.doi.org/10.1093/carcin/22.4.579.
122. Li M, Fang X, Baker DJ, Guo L, Gao X, Wei Z, Han S, van Deursen JM, Zhang P. 2010. The ATM–p53 pathway suppresses aneuploidy-induced tumorigenesis. Proceedings of the National Academy of Sciences. 107(32):14188–14193. doi:10.1073/pnas.1005960107. http://dx.doi.org/10.1073/pnas.1005960107.
123. Thompson SL, Compton DA. 2010. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. Journal of Cell Biology. 188(3):369–381. doi:10.1083/jcb.200905057. http://dx.doi.org/10.1083/jcb.200905057.
124. Jones KT. 2007. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Human Reproduction Update. 14(2):143–158. doi:10.1093/humupd/dmm043. http://dx.doi.org/10.1093/humupd/dmm043.
125. Hassold T, Hunt P. 2001. To err (meiotically) is human: the genesis of human aneuploidy. Nature Reviews Genetics. 2(4):280–291. doi:10.1038/35066065. http://dx.doi.org/10.1038/35066065.
126. Fisher D, DiPietro A, Murdison KA, Lemieux CA. 2012. Full Monosomy 21: Echocardiographic Findings in the Third Molecularly Confirmed Case. Pediatric Cardiology. 34(3):733–735. doi:10.1007/s00246-012-0334-4. http://dx.doi.org/10.1007/s00246-012-0334-4.
127. Nagaoka SI, Hassold TJ, Hunt PA. 2012. Human aneuploidy: mechanisms and new insights into an age-old problem. Nature Reviews Genetics. 13(7):493–504. doi:10.1038/nrg3245. http://dx.doi.org/10.1038/nrg3245.
128. Carrell D. 2003. Elevated sperm chromosome aneuploidy and apoptosis in patients with unexplained recurrent pregnancy loss. Obstetrics & Gynecology. 101(6):1229–1235. doi:10.1016/s0029-7844(03)00339-9. http://dx.doi.org/10.1016/s0029-7844(03)00339-9.
129. Goddijn M, Leschot NJ. 2000. Genetic aspects of miscarriage. Best Practice & Research Clinical Obstetrics & Gynaecology. 14(5):855–865. doi:10.1053/beog.2000.0124. http://dx.doi.org/10.1053/beog.2000.0124.
130. Munné S, Sandalinas M, Escudero T, Velilla E, Walmsley R, Sadowy S, Cohen J, Sable D. 2003. Improved implantation after preimplantation genetic diagnosis of aneuploidy. Reproductive BioMedicine Online. 7(1):91–97. doi:10.1016/s1472-6483(10)61735-x. http://dx.doi.org/10.1016/s1472-6483(10)61735-x.
131. Templado C, Uroz L, Estop A. 2013. New insights on the origin and relevance of aneuploidy in human spermatozoa. MHR: Basic science of reproductive medicine. 19(10):634–643. doi:10.1093/molehr/gat039. http://dx.doi.org/10.1093/molehr/gat039.
132. Wilch ES, Morton CC. 2018. Historical and Clinical Perspectives on Chromosomal Translocations. Advances in Experimental Medicine and Biology.:1–14. doi:10.1007/978-981-13-0593-1_1. http://dx.doi.org/10.1007/978-981-13-0593-1_1.
133. Soussi T, Lozano G. 2005. p53 mutation heterogeneity in cancer. Biochemical and Biophysical Research Communications. 331(3):834–842. doi:10.1016/j.bbrc.2005.03.190. http://dx.doi.org/10.1016/j.bbrc.2005.03.190.
134. Prior IA, Hood FE, Hartley JL. 2020. The Frequency of Ras Mutations in Cancer. Cancer Research. 80(14):2969–2974. doi:10.1158/0008-5472.can-19-3682. http://dx.doi.org/10.1158/0008-5472.can-19-3682.
135. Jego N, Thomas G, Hamelin R. 1993. Short direct repeats flanking deletions, and duplicating insertions in p53 gene in human cancers. Oncogene. 8(1):209–13. [accessed 2024 Feb 6]. https://pubmed.ncbi.nlm.nih.gov/8380918/.
136. Gascoigne KE, Cheeseman IM. 2013. Induced dicentric chromosome formation promotes genomic rearrangements and tumorigenesis. Chromosome Research. 21(4):407–418. doi:10.1007/s10577-013-9368-6. http://dx.doi.org/10.1007/s10577-013-9368-6.
137. Rabbitts TH. 1994. Chromosomal translocations in human cancer. Nature. 372(6502):143–149. doi:10.1038/372143a0. http://dx.doi.org/10.1038/372143a0.
138. Nussenzweig A, Nussenzweig MC. 2010. Origin of Chromosomal Translocations in Lymphoid Cancer. Cell. 141(1):27–38. doi:10.1016/j.cell.2010.03.016. http://dx.doi.org/10.1016/j.cell.2010.03.016.
139. Bhatia A, Kumar Y. 2012. Cancer cell micronucleus: an update on clinical and diagnostic applications. APMIS. 121(7):569–581. doi:10.1111/apm.12033. http://dx.doi.org/10.1111/apm.12033.
140. Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D. 2012. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 482(7383):53–58. doi:10.1038/nature10802. http://dx.doi.org/10.1038/nature10802.
141. Ben-David U, Amon A. 2019. Context is everything: aneuploidy in cancer. Nature Reviews Genetics. 21(1):44–62. doi:10.1038/s41576-019-0171-x. http://dx.doi.org/10.1038/s41576-019-0171-x.
142. Ly P, Brunner SF, Shoshani O, Kim DH, Lan W, Pyntikova T, Flanagan AM, Behjati S, Page DC, Campbell PJ, et al. 2019. Chromosome segregation errors generate a diverse spectrum of simple and complex genomic rearrangements. Nature Genetics. 51(4):705–715. doi:10.1038/s41588-019-0360-8. http://dx.doi.org/10.1038/s41588-019-0360-8.
143. Othman OEMS, Aly MS, Nahas SME. 2001. Aneuploidy in Workers Occupationally Exposed to Electromagnetic Field Detected by FISH. CYTOLOGIA. 66(2):117–125. doi:10.1508/cytologia.66.117. http://dx.doi.org/10.1508/cytologia.66.117.
144. Helbock HJ, Beckman KB, Shigenaga MK, Walter PB, Woodall AA, Yeo HC, Ames BN. 1998. DNA oxidation matters: The HPLC–electrochemical detection assay of 8-oxo-deoxyguanosine and 8-oxo-guanine. Proceedings of the National Academy of Sciences. 95(1):288–293. doi:10.1073/pnas.95.1.288. http://dx.doi.org/10.1073/pnas.95.1.288.
145. Nakamura J, Walker VE, Upton PB, Chiang SY, Kow YW, Swenberg JA. 1998. Highly sensitive apurinic/apyrimidinic site assay can detect spontaneous and chemically induced depurination under physiological conditions. Cancer research. 58(2):222–5. [accessed 2024 Feb 6]. https://pubmed.ncbi.nlm.nih.gov/9443396/.
146. Hossain M, Lin Y, Yan S. 2018. Single-Strand Break End Resection in Genome Integrity: Mechanism and Regulation by APE2. International Journal of Molecular Sciences. 19(8):2389. doi:10.3390/ijms19082389. http://dx.doi.org/10.3390/ijms19082389.
147. Vilenchik MM, Knudson AG. 2003. Endogenous DNA double-strand breaks: Production, fidelity of repair, and induction of cancer. Proceedings of the National Academy of Sciences. 100(22):12871–12876. doi:10.1073/pnas.2135498100. http://dx.doi.org/10.1073/pnas.2135498100.
148. Gürler HŞ, Bilgici B, Akar AK, Tomak L, Bedir A. 2014. Increased DNA oxidation (8-OHdG) and protein oxidation (AOPP) by low level electromagnetic field (2.45 GHz) in rat brain and protective effect of garlic. International Journal of Radiation Biology. 90(10):892–896. doi:10.3109/09553002.2014.922717. http://dx.doi.org/10.3109/09553002.2014.922717.
149. Zhang Y, Zhang D, Zhu B, Zhang H, Sun Y, Sun C. 2016. Effects of dietary green tea polyphenol supplementation on the health of workers exposed to high-voltage power lines. Environmental Toxicology and Pharmacology. 46:183–187. doi:10.1016/j.etap.2016.07.016. http://dx.doi.org/10.1016/j.etap.2016.07.016.
150. Lai H, Singh NP. 2004. Magnetic-field-induced DNA strand breaks in brain cells of the rat. Environmental Health Perspectives. 112(6):687–694. doi:10.1289/ehp.6355. http://dx.doi.org/10.1289/ehp.6355.
151. Rageh MM, EL-Gebaly RH, El-Bialy NS. 2012. Assessment of Genotoxic and Cytotoxic Hazards in Brain and Bone Marrow Cells of Newborn Rats Exposed to Extremely Low-Frequency Magnetic Field. Journal of Biomedicine and Biotechnology. 2012:1–7. doi:10.1155/2012/716023. http://dx.doi.org/10.1155/2012/716023.
152. Ding S-S, Sun P, Tian H, Huo Y-W, Wang L-R, Han Y, Zhang Z, Liu X, Xing J-P. 2018. Association between daily exposure to electromagnetic radiation from 4G smartphone and 2.45-GHz wi-fi and oxidative damage to semen of males attending a genetics clinic: a primary study. International Journal of Clinical and Experimental Medicine. 11(3):2821–2830. https://www.ijcem.com/files/ijcem0063001.pdf.
153. Kumar S, Nirala JP, Behari J, Paulraj R. 2014. Effect of electromagnetic irradiation produced by 3G mobile phone on male rat reproductive system in a simulated scenario. Indian journal of experimental biology. 52(9):890–7. [accessed 2024 Feb 6]. https://pubmed.ncbi.nlm.nih.gov/25241589/.
154. Bioinitiative. 2022. Henry Lai’s Research Summaries. [accessed 2024 Mar 27]. https://bioinitiative.org/research-summaries/.
155. Yakymenko I, Tsybulin O, Sidorik E, Henshel D, Kyrylenko O, Kyrylenko S. 2015. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagnetic Biology and Medicine. 35(2):186–202. doi:10.3109/15368378.2015.1043557. http://dx.doi.org/10.3109/15368378.2015.1043557.
156. Alchalabi ASH, Aklilu E, Aziz AR, Malek F, Ronald SH, Khan MA. 2016. Different periods of intrauterine exposure to electromagnetic field: Influence on female rats’ fertility, prenatal and postnatal development. Asian Pacific Journal of Reproduction. 5(1):14–23. doi:10.1016/j.apjr.2015.12.003. http://dx.doi.org/10.1016/j.apjr.2015.12.003.
157. Khaki A. 2013. Effect of Ocimum basilicum on ovary tissue histopathology after exposure to electromagnetic fields (EMF) in rats. African Journal of Pharmacy and Pharmacology. 7(25):1703–1706. doi:10.5897/ajpp12.1073. http://dx.doi.org/10.5897/ajpp12.1073.
158. Sangun O, Dundar B, Darici H, Comlekci S, Doguc DK, Celik S. 2014. The effects of long-term exposure to a 2450 MHz electromagnetic field on growth and pubertal development in female Wistar rats. Electromagnetic Biology and Medicine. 34(1):63–71. doi:10.3109/15368378.2013.871619. http://dx.doi.org/10.3109/15368378.2013.871619.
159. Gao Q-H, Cai Q, Fan Y. 2017. Beneficial effect of catechin and epicatechin on cognitive impairment and oxidative stress induced by extremely low frequency electromagnetic field. Journal of Food Biochemistry. 41(6):e12416. doi:10.1111/jfbc.12416. http://dx.doi.org/10.1111/jfbc.12416.
160. Yahyazadeh A, Kıvrak EG, Koç GE. 2021. Protective effect of melatonin on the rat lung following exposure to 900-MHz electromagnetic field: a stereological and histopathological study. Journal of Experimental and Clinical Medicine. 38(2):55–60. doi:10.52142/omujecm.38.2.1. http://dx.doi.org/10.52142/omujecm.38.2.1.
161. Gharib OA. 2011. Role of Kombucha Tea in the Control of EMF 950 MHz Induced Injury in Rat Heart and Lung Organs. Asian Journal of Pharmaceutical and Biological. 1(3):281–288. https://www.researchgate.net/publication/232282167/.
162. Baltaci AK, Mogulkoc R, Salbacak A, Celik I, Sivrikaya A. 2012. The role of zinc supplementation in the inhibition of tissue damage caused by exposure to electromagnetic field in rat lung and liver tissues. Bratislava Medical Journal. 113(07):400–403. doi:10.4149/bll_2012_090. http://dx.doi.org/10.4149/bll_2012_090.
163. Masoumi A, Karbalaei N, Mortazavi SMJ, Shabani M. 2018. Radiofrequency radiation emitted from Wi-Fi (2.4 GHz) causes impaired insulin secretion and increased oxidative stress in rat pancreatic islets. International Journal of Radiation Biology. 94(9):850–857. doi:10.1080/09553002.2018.1490039. http://dx.doi.org/10.1080/09553002.2018.1490039.
164. TOPAL Z, HANCI H, MERCANTEPE T, EROL HS, KELEŞ ON, KAYA H, MUNGAN S, ODACI E. 2015. The effects of prenatal long-duration exposure to 900-MHz electromagnetic field on the 21-day-old newborn male rat liver. TURKISH JOURNAL OF MEDICAL SCIENCES. 45:291–297. doi:10.3906/sag-1404-168. http://dx.doi.org/10.3906/sag-1404-168.
165. Bedir R, Tumkaya L, Mercantepe T, Yilmaz A. 2018. Pathological Findings Observed in the Kidneys of Postnatal Male Rats Exposed to the 2100 MHz Electromagnetic Field. Archives of Medical Research. 49(7):432–440. doi:10.1016/j.arcmed.2018.12.010. http://dx.doi.org/10.1016/j.arcmed.2018.12.010.
166. Oktem F, Ozguner F, Mollaoglu H, Koyu A, Uz E. 2005. Oxidative Damage in the Kidney Induced by 900-MHz-Emitted Mobile Phone: Protection by Melatonin. Archives of Medical Research. 36(4):350–355. doi:10.1016/j.arcmed.2005.03.021. http://dx.doi.org/10.1016/j.arcmed.2005.03.021.
167. Balci M, Devrim E, Durak I. 2007. Effects of Mobile Phones on Oxidant/Antioxidant Balance in Cornea and Lens of Rats. Current Eye Research. 32(1):21–25. doi:10.1080/02713680601114948. http://dx.doi.org/10.1080/02713680601114948.
168. Zothansiama, Zosangzuali M, Lalramdinpuii M, Jagetia GC. 2017. Impact of radiofrequency radiation on DNA damage and antioxidants in peripheral blood lymphocytes of humans residing in the vicinity of mobile phone base stations. Electromagnetic Biology and Medicine. 36(3):295–305. doi:10.1080/15368378.2017.1350584. http://dx.doi.org/10.1080/15368378.2017.1350584.
169. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, et al. 2018. Oxidative stress, aging, and diseases. Clinical Interventions in Aging. Volume 13:757–772. doi:10.2147/cia.s158513. http://dx.doi.org/10.2147/cia.s158513.
170. Zuo L, He F, Sergakis GG, Koozehchian MS, Stimpfl JN, Rong Y, Diaz PT, Best TM. 2014. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. American Journal of Physiology-Lung Cellular and Molecular Physiology. 307(3):L205–L218. doi:10.1152/ajplung.00330.2013. http://dx.doi.org/10.1152/ajplung.00330.2013.
171. Albano E. 2006. Alcohol, oxidative stress and free radical damage. Proceedings of the Nutrition Society. 65(3):278–290. doi:10.1079/pns2006496. http://dx.doi.org/10.1079/pns2006496.
172. Dennery PA. 2007. Effects of oxidative stress on embryonic development. Birth Defects Research Part C: Embryo Today: Reviews. 81(3):155–162. doi:10.1002/bdrc.20098. http://dx.doi.org/10.1002/bdrc.20098.
173. Al-Gubory KH, Fowler PA, Garrel C. 2010. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. The International Journal of Biochemistry & Cell Biology. 42(10):1634–1650. doi:10.1016/j.biocel.2010.06.001. http://dx.doi.org/10.1016/j.biocel.2010.06.001.
174. Hempstock J, Jauniaux E, Greenwold N, Burton GJ. 2003. The contribution of placental oxidative stress to early pregnancy failure. Human Pathology. 34(12):1265–1275. doi:10.1016/j.humpath.2003.08.006. http://dx.doi.org/10.1016/j.humpath.2003.08.006.
175. Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu Ö, Durak İ. 2007. Role of Oxidative Stress in Intrauterine Growth Restriction. Gynecologic and Obstetric Investigation. 64(4):187–192. doi:10.1159/000106488. http://dx.doi.org/10.1159/000106488.
176. Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. 2017. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. Journal of clinical and diagnostic research : JCDR. 11(5):IE01–IE05. doi:10.7860/JCDR/2017/23927.9886. [accessed 2024 Mar 27]. https://pubmed.ncbi.nlm.nih.gov/28658802/.
177. Hung J-H, Chen C-Y, Omar HA, Huang K-Y, Tsao C-C, Chiu C-C, Chen Y-L, Chen P-H, Teng Y-N. 2015. Reactive oxygen species mediate Terbufos-induced apoptosis in mouse testicular cell lines via the modulation of cell cycle and pro-apoptotic proteins. Environmental Toxicology. 31(12):1888–1898. doi:10.1002/tox.22190. http://dx.doi.org/10.1002/tox.22190.
178. Guo Z, Wang X, Zhang P, Sun F, Chen Z, Ma W, Meng F, Hao H, Shang X. 2022. Silica nanoparticles cause spermatogenesis dysfunction in mice via inducing cell cycle arrest and apoptosis. Ecotoxicology and Environmental Safety. 231:113210. doi:10.1016/j.ecoenv.2022.113210. http://dx.doi.org/10.1016/j.ecoenv.2022.113210.
179. Guvvala PR, Sellappan S, Parameswaraiah RJ. 2016. Impact of arsenic(V) on testicular oxidative stress and sperm functional attributes in Swiss albino mice. Environmental Science and Pollution Research. 23(18):18200–18210. doi:10.1007/s11356-016-6870-3. http://dx.doi.org/10.1007/s11356-016-6870-3.
180. Yeh Y-C, Lai Hui-Chin, Ting C-T, Lee W-L, Wang L-C, Wang K-Y, Lai Hui-Chun, Liu T-J. 2007. Protection by doxycycline against doxorubicin-induced oxidative stress and apoptosis in mouse testes. Biochemical Pharmacology. 74(7):969–980. doi:10.1016/j.bcp.2007.06.031. http://dx.doi.org/10.1016/j.bcp.2007.06.031.
181. H. D, M.H. C. 2012. Ovarian Follicular Atresia. Basic Gynecology - Some Related Issues. doi:10.5772/32465. http://dx.doi.org/10.5772/32465.
182. Gupta RK, Miller KP, Babus JK, Flaws JA. 2006. Methoxychlor Inhibits Growth and Induces Atresia of Antral Follicles through an Oxidative Stress Pathway. Toxicological Sciences. 93(2):382–389. doi:10.1093/toxsci/kfl052. http://dx.doi.org/10.1093/toxsci/kfl052.
183. Wang W, Craig ZR, Basavarajappa MS, Gupta RK, Flaws JA. 2012. Di (2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicology and Applied Pharmacology. 258(2):288–295. doi:10.1016/j.taap.2011.11.008. http://dx.doi.org/10.1016/j.taap.2011.11.008.
184. Dai X-X, Duan X, Cui X-S, Kim N-H, Xiong B, Sun S-C. 2015. Melamine Induces Oxidative Stress in Mouse Ovary. Sun Q-Y, editor. PLOS ONE. 10(11):e0142564. doi:10.1371/journal.pone.0142564. http://dx.doi.org/10.1371/journal.pone.0142564.
185. Wu J, Yuan M, Song Y, Sun F, Han X. 2015. MC-LR Exposure Leads to Subfertility of Female Mice and Induces Oxidative Stress in Granulosa Cells. Toxins. 7(12):5212–5223. doi:10.3390/toxins7124872. http://dx.doi.org/10.3390/toxins7124872.
186. Maiti P, Singh SB, Mallick B, Muthuraju S, Ilavazhagan G. 2008. High altitude memory impairment is due to neuronal apoptosis in hippocampus, cortex and striatum. Journal of Chemical Neuroanatomy. 36(3–4):227–238. doi:10.1016/j.jchemneu.2008.07.003. http://dx.doi.org/10.1016/j.jchemneu.2008.07.003.
187. Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S. 2001. Impairment of Learning and Memory in Rats Caused by Oxidative Stress and Aging, and Changes in Antioxidative Defense Systems. Annals of the New York Academy of Sciences. 928(1):168–175. doi:10.1111/j.1749-6632.2001.tb05646.x. http://dx.doi.org/10.1111/j.1749-6632.2001.tb05646.x.
188. Treviño S, Aguilar‐Alonso P, Flores Hernandez JA, Brambila E, Guevara J, Flores G, Lopez‐Lopez G, Muñoz‐Arenas G, Morales‐Medina JC, Toxqui V, et al. 2015. A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse. 69(9):421–433. doi:10.1002/syn.21832. http://dx.doi.org/10.1002/syn.21832.
189. MANDA K, UENO M, ANZAI K. 2008. Memory impairment, oxidative damage and apoptosis induced by space radiation: Ameliorative potential of α-lipoic acid. Behavioural Brain Research. 187(2):387–395. doi:10.1016/j.bbr.2007.09.033. http://dx.doi.org/10.1016/j.bbr.2007.09.033.
190. Joseph N, Zhang-James Y, Perl A, Faraone SV. 2013. Oxidative Stress and ADHD. Journal of Attention Disorders. 19(11):915–924. doi:10.1177/1087054713510354. http://dx.doi.org/10.1177/1087054713510354.
191. James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA. 2004. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. The American Journal of Clinical Nutrition. 80(6):1611–1617. doi:10.1093/ajcn/80.6.1611. http://dx.doi.org/10.1093/ajcn/80.6.1611.
192. Carrı̀ MT, Ferri A, Cozzolino M, Calabrese L, Rotilio G. 2003. Neurodegeneration in amyotrophic lateral sclerosis: the role of oxidative stress and altered homeostasis of metals. Brain Research Bulletin. 61(4):365–374. doi:10.1016/s0361-9230(03)00179-5. http://dx.doi.org/10.1016/s0361-9230(03)00179-5.
193. Dumont E, Monari A. 2015. Understanding DNA under oxidative stress and sensitization: the role of molecular modeling. Frontiers in Chemistry. 3. doi:10.3389/fchem.2015.00043. http://dx.doi.org/10.3389/fchem.2015.00043.
194. Dedon PC, Plastaras JP, Rouzer CA, Marnett LJ. 1998. Indirect mutagenesis by oxidative DNA damage: Formation of the pyrimidopurinone adduct of deoxyguanosine by base propenal. Proceedings of the National Academy of Sciences. 95(19):11113–11116. doi:10.1073/pnas.95.19.11113. http://dx.doi.org/10.1073/pnas.95.19.11113.
195. Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. 2011. Role of oxidative stress and DNA damage in human carcinogenesis. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 711(1–2):193–201. doi:10.1016/j.mrfmmm.2010.12.016. http://dx.doi.org/10.1016/j.mrfmmm.2010.12.016.
196. Klaunig JE, Kamendulis LM, Hocevar BA. 2009. Oxidative Stress and Oxidative Damage in Carcinogenesis. Toxicologic Pathology. 38(1):96–109. doi:10.1177/0192623309356453. http://dx.doi.org/10.1177/0192623309356453.
197. Talpaert-Borlè M. 1987. Formation, detection and repair of AP sites. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 181(1):45–56. doi:10.1016/0027-5107(87)90286-7. http://dx.doi.org/10.1016/0027-5107(87)90286-7.
198. Gelot C, Magdalou I, Lopez B. 2015. Replication Stress in Mammalian Cells and Its Consequences for Mitosis. Genes. 6(2):267–298. doi:10.3390/genes6020267. http://dx.doi.org/10.3390/genes6020267.
199. Shibata A, Jeggo PA. 2020. Canonical DNA non-homologous end-joining; capacity versus fidelity. The British Journal of Radiology. 93(1115):20190966. doi:10.1259/bjr.20190966. http://dx.doi.org/10.1259/bjr.20190966.
200. Norppa H. 2003. What do human micronuclei contain? Mutagenesis. 18(3):221–233. doi:10.1093/mutage/18.3.221. http://dx.doi.org/10.1093/mutage/18.3.221.
201. Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D. 2012. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 482(7383):53–58. doi:10.1038/nature10802. http://dx.doi.org/10.1038/nature10802.
202. Gascoigne KE, Cheeseman IM. 2013. Induced dicentric chromosome formation promotes genomic rearrangements and tumorigenesis. Chromosome Research. 21(4):407–418. doi:10.1007/s10577-013-9368-6. http://dx.doi.org/10.1007/s10577-013-9368-6.
203. Popp HD, Brendel S, Hofmann W-K, Fabarius A. 2017. Immunofluorescence Microscopy of γH2AX and 53BP1 for Analyzing the Formation and Repair of DNA Double-strand Breaks. Journal of Visualized Experiments.(129). doi:10.3791/56617. http://dx.doi.org/10.3791/56617.
204. Wilhelm T, Olziersky A-M, Harry D, De Sousa F, Vassal H, Eskat A, Meraldi P. 2019. Mild replication stress causes chromosome mis-segregation via premature centriole disengagement. Nature Communications. 10(1). doi:10.1038/s41467-019-11584-0. http://dx.doi.org/10.1038/s41467-019-11584-0.
205. Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED. 2001. Merotelic Kinetochore Orientation Is a Major Mechanism of Aneuploidy in Mitotic Mammalian Tissue Cells. The Journal of Cell Biology. 153(3):517–528. doi:10.1083/jcb.153.3.517. http://dx.doi.org/10.1083/jcb.153.3.517.
206. Pesnya DS, Romanovsky AV. 2013. Comparison of cytotoxic and genotoxic effects of plutonium-239 alpha particles and mobile phone GSM 900 radiation in the Allium cepa test. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 750(1–2):27–33. doi:10.1016/j.mrgentox.2012.08.010. http://dx.doi.org/10.1016/j.mrgentox.2012.08.010.
207. D’Ambroslo G, Scaglione A, Berardino DD, Lioi MB, Iannuzzi L, Mostacciuolo E, Scarfi MR. 1985. Chromosomal Aberrations Induced by Elf Electric Fields. Journal of Bioelectricity. 4(1):279–284. doi:10.3109/15368378509040379. http://dx.doi.org/10.3109/15368378509040379.
208. Mairs RJ, Hughes K, Fitzsimmons S, Prise KM, Livingstone A, Wilson L, Baig N, Clark AM, Timpson A, Patel G, et al. 2007. Microsatellite analysis for determination of the mutagenicity of extremely low-frequency electromagnetic fields and ionising radiation in vitro. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 626(1–2):34–41. doi:10.1016/j.mrgentox.2006.08.005. http://dx.doi.org/10.1016/j.mrgentox.2006.08.005.
209. Mashevich M, Folkman D, Kesar A, Barbul A, Korenstein R, Jerby E, Avivi L. 2003. Exposure of human peripheral blood lymphocytes to electromagnetic fields associated with cellular phones leads to chromosomal instability. Bioelectromagnetics. 24(2):82–90. doi:10.1002/bem.10086. http://dx.doi.org/10.1002/bem.10086.
210. Manikowska-Czerska E, Czerskl P, Leach WM. 1985. Effects of 2.45 GHz microwaves on meiotic chromosomes of male CBA/CAY mice. Journal of Heredity. 76(1):71–73. doi:10.1093/oxfordjournals.jhered.a110027. http://dx.doi.org/10.1093/oxfordjournals.jhered.a110027.
211. Nagaoka SI, Hassold TJ, Hunt PA. 2012. Human aneuploidy: mechanisms and new insights into an age-old problem. Nature Reviews Genetics. 13(7):493–504. doi:10.1038/nrg3245. http://dx.doi.org/10.1038/nrg3245.
212. Balamuralikrishnan B, Balachandar V, Kumar SS, Stalin N, Varsha P, Devi SM, Arun M, Manikantan P, Venkatesan C, Sasikala K, et al. 2012. Evaluation of Chromosomal Alteration in Electrical Workers Occupationally Exposed to Low Frequency of Electro Magnetic Field (EMFs) in Coimbatore Population, India. Asian Pacific Journal of Cancer Prevention. 13(6):2961–2966. doi:10.7314/apjcp.2012.13.6.2961. http://dx.doi.org/10.7314/apjcp.2012.13.6.2961.
213. Gandhi G, Singh P. 2005. Cytogenetic Damage in Mobile Phone Users: Preliminary Data. International Journal of Human Genetics. 5(4):259–265. doi:10.1080/09723757.2005.11885936. http://dx.doi.org/10.1080/09723757.2005.11885936.
214. Estécio MRH, Silva AE. 2002. Alterações cromossômicas causadas pela radiação dos monitores de vídeo de computadores. Revista de Saúde Pública. 36(3):330–336. doi:10.1590/s0034-89102002000300012. http://dx.doi.org/10.1590/s0034-89102002000300012.
215. Nordenson I, Hansson Mild K, Nordstr�m S, Sweins A, Birke E. 1984. Clastogenic effects in human lymphocytes of power frequency electric fields: In vivo and in vitro studies. Radiation and Environmental Biophysics. 23(3):191–201. doi:10.1007/bf01213221. http://dx.doi.org/10.1007/bf01213221.

