Cell Proliferation

Next, I will explain a pathway of cell proliferation caused by reactive oxygen species (ROS).
I will then present studies showing that EMFs do indeed cause cell proliferation.

Table of ContentsAll_Pages
General Pathway of Cell Proliferation
Generally speaking, when a resting cell receives a protein called a growth factor, it enters the cell cycle and initiates the series of processes for cell proliferation.
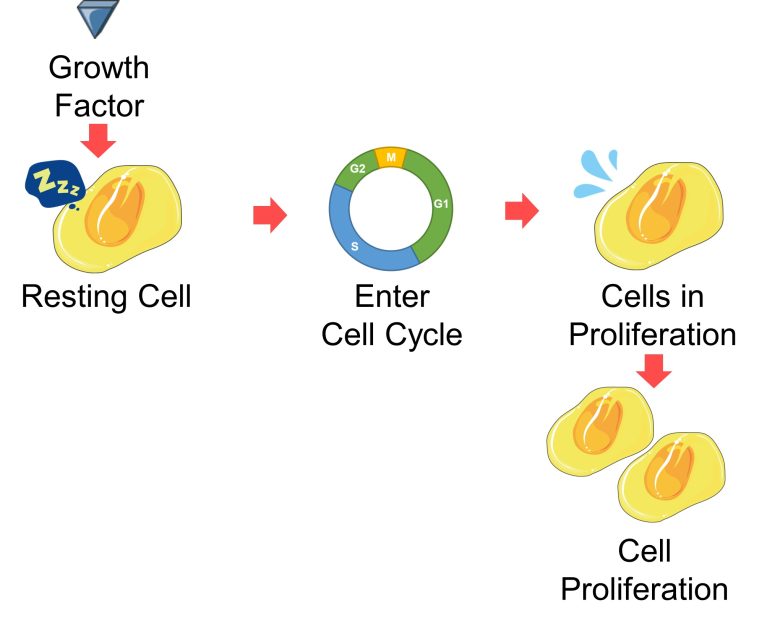
Below are the signaling pathways within the cell from receiving the growth factor to entering the cell cycle.
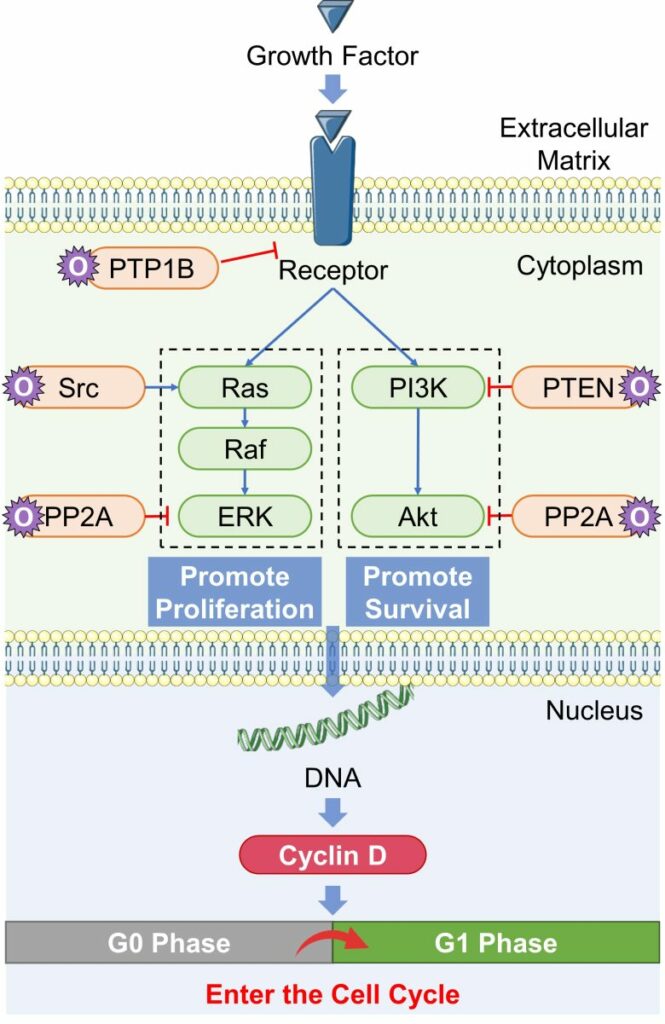
General Pathway of Cell Proliferation
( Cited and Modified from Scaltriti and Baselga 2006 )
- A receptor in the cell membrane binds to a growth factor and activates.
- The activated receptor subsequently activate a pathway that promote proliferation (Ras-Raf-ERK).
- It also activates a pathway that promotes survival (PI3K-Akt).
- Proteins downstream of these pathways act on DNA to stimulate the production of proteins necessary for cell proliferation, such as cyclin D.
- When cyclin D increases to a certain amount, the cell that were in the resting phase (G0 phase) enter the cell cycle (G1 phase) and begin cell proliferation.
- ROS affect some of the proteins involved in cell proliferation (orange in the figure above), leading to cell proliferation.
That is, an increase in ROS can promote cell proliferation even in the absence of growth factors.
Cell Proliferation by ROS
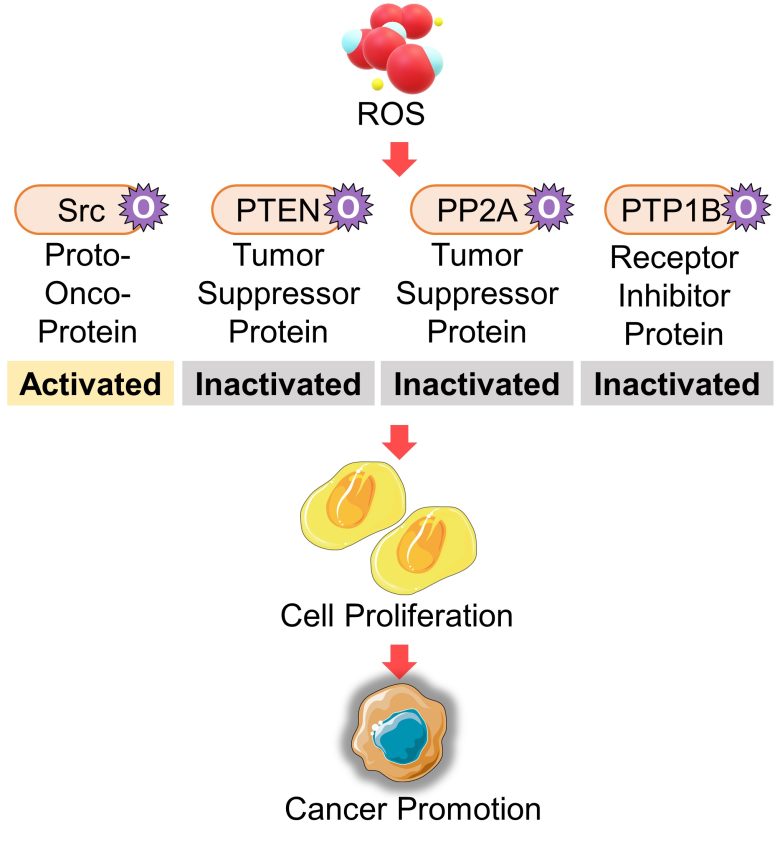
ROS interfere with cell proliferation and survival pathways, particularly by activating proteins of proto-oncogenes and inactivating proteins of tumor suppressor genes.
Activation of the Proto-Oncogene Src
Proteins of the proto-oncogene Src are oxidized and then activated by ROS. This oxidative pathway is considered to be required for the tumorigenic ability of Src. (Giannoni et al. 2005)
Oxidative stress-induced activation of the Ras-Raf-ERK pathway, which promotes proliferation, is triggered by Src oxidation. (Aikawa et al. 1997)
That is, an increase in ROS activates proteins of the proto-oncogene Src, which activates the pathway that promotes cell proliferation.
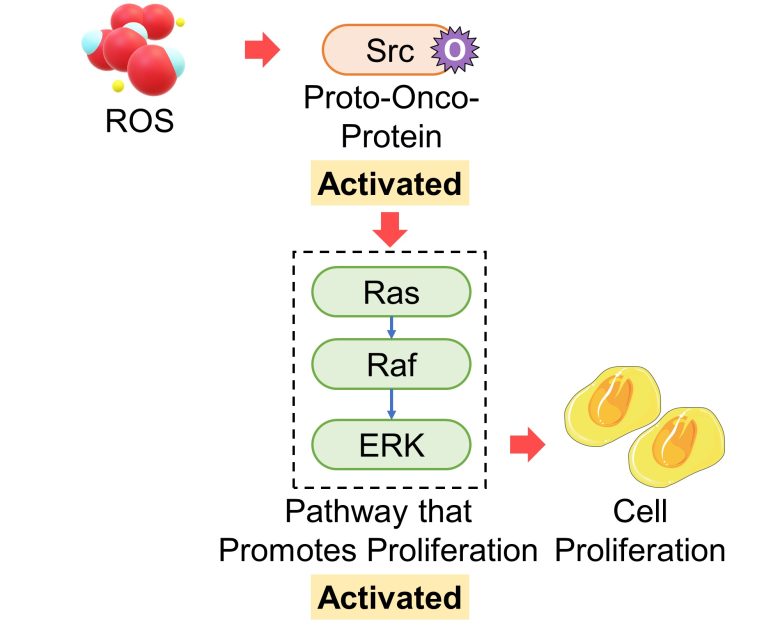
As mentioned in the mutation section, Ras, which is activated by Src, is also a well-known proto-oncogene.
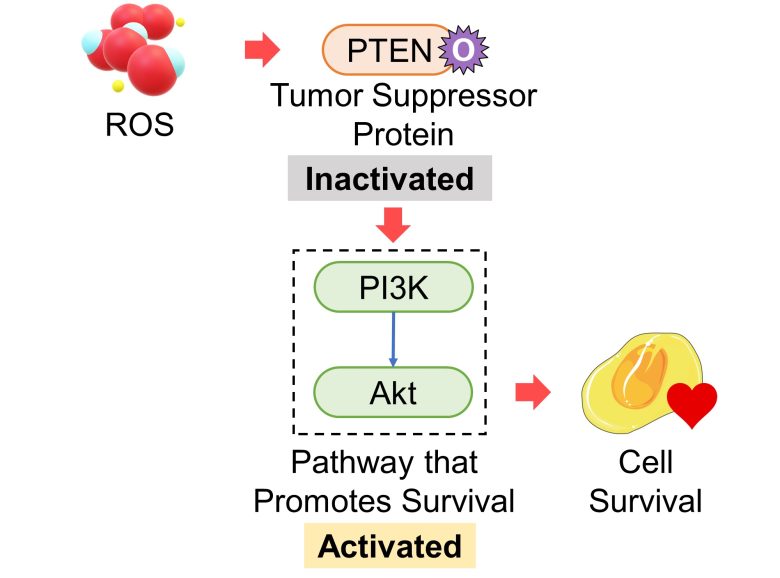
Inactivation of the Tumore Suppressor Gene PTEN
Proteins of the cancer-suppressor gene PTEN inhibit the PI3K-Akt pathway, which promotes survival. The tumor suppressive ability of PTEN is thought to be due to this regulation. (Stambolic et al. 1998)
PTEN is oxidized and then inactivated by ROS, which subsequently activates the PI3K-Akt pathway. (Wu et al. 2013)
That is, an increase in ROS inactivates proteins of the tumor suppressor gene PTEN, which activates the pathway that promotes cell survival.
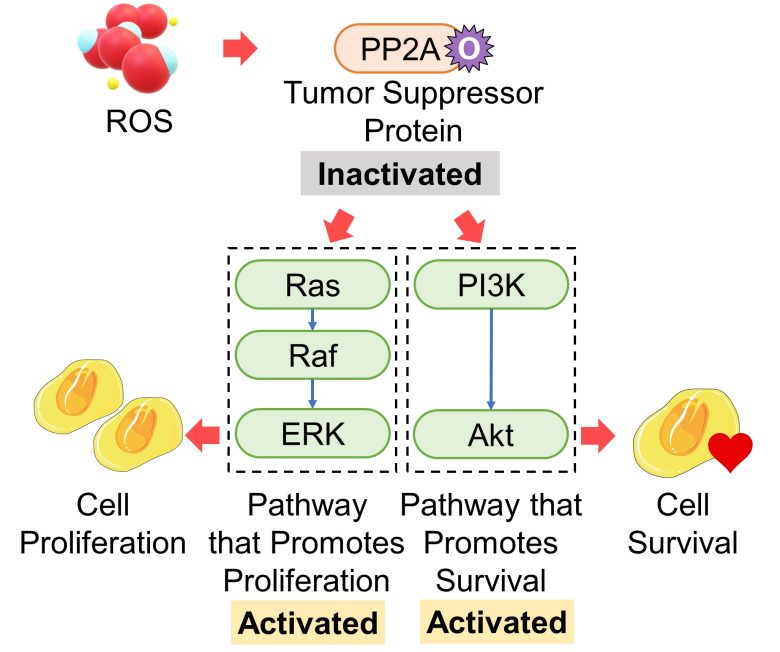
Inactivation of the Tumor Suppressor Gene PP2A
Proteins of the tumor suppressor gene PP2A suppress cancer primarily by inactivating ERK and Akt, i.e., by inhibiting the Ras-Raf-ERK pathway, which promotes proliferation, and the PI3K-Akt pathway, which promotes survival. (Ciccone et al. 2015)
PP2A is oxidized and then inactivated by oxidative stress. (Nakahata and Morishita 2014, Shimura et al. 2015)
That is, an increase in ROS inactivates proteins of the tumor suppressor gene PP2A, which activates the pathways that promote cell proliferation and cell survival.
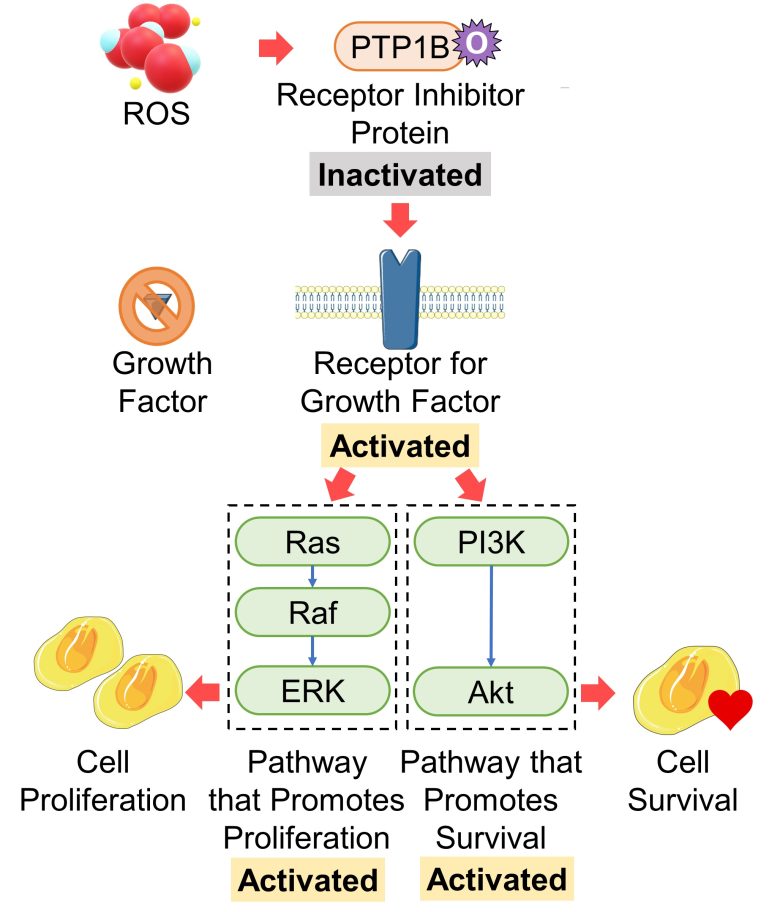
Activation of the Receptor Inhibitor Protein PP1B
The activation of growth factor receptors is suppressed by an inhibitory protein called PTP1B. When PTP1B is oxidized and then inactivated by ROS, this inhibitory effect is weakened. (Chiarugi and Cirri 2003)
In fact, it was found that oxidative stress activated growth factor receptors even in the absence of growth factors. (Meves et al. 2001)
That is, an increase in ROS activates growth factor receptors (probably by oxidation and then inactivation of the receptor inhibitor protein PTP1B), which activates the pathways that promote cell proliferation and cell survival.
Consequences of Cell Proliferation
Cancer Promotion
Taking the above pathway as a whole, it follows that ROS can trigger cell proliferation by oxidizing at least four proteins: Src, PTEN, PP2A, and PTP1B.
A possible health effect related to this is cancer promotion.
Cancer By EMFs
And in fact, EMF exposure has been shown to increase wide-ranging cancers, including leukemia, lymphoma, brain tumors, breast cancer, and testicular cancer. These studies can be found on page 3 of the following article.
Cancer (Page 3)
Leukemia and Lymphoma Brain Tumors Breast Cancer Testicular Cancer Other Cancers
It has been shown that EMF exposure increases various types of cancer, including leukemia (especially lymphoid leukemia), lymphoma, breast cancer, testicular cancer, lung cancer, and pancreatic cancer. In recent years, these cancers are on the rise, and EMFs are likely to be one of the… Read the Full Article
Cell Proliferation by EMFs
Here, I will present studies showing that cell proliferation occurred with EMF exposure. In particular, cancer cells have been shown to proliferate well.
Martínez et al. 2012
Human cancer cells of neuroblastoma, a cancer most commonly found in infants and toddlers, were exposed to 50 Hz ELF-EMF with a strength of 100 μT for 63 hours, either continuously or intermittently.
As a result, cell count increased with the intermittent exposure, meaning that the intermittent EMF exposure promoted proliferation of the cancer cells.
On the other hand, continuous exposure did not change cell count.
Furthermore, it was also found that the EMFs promoted cell proliferation by activating the ERK1/2 pathway.
Increase in Cell Count
The cell counts increased due to the intermittent exposure to ELF-EMFs.
Velizarov et al. 1999
Transformed, i.e., turned into cancerous, human amniotic epithelial cells, cells from the placenta, were exposed to cell phone EMFs at a culture-medium average SAR of 0.002 W/kg for only 30 minutes.
As a result, cell count increased, meaning that the cell phone EMFs promoted proliferation of the cancerous cells.
Also, two different cell incubators were used in this experiment, one at 35°C and the other at 39°C, and there was no effect of temperature change on cell proliferation.
Therefore, cell proliferation caused by EMFs is likely to derive from ROS rather than thermal effects.
Increase in Cell Count
Tang and Zhao 1999
Mouse osteoblasts, cells that synthesize bone, were exposed to 50Hz ELF-EMFs wiith a strength of 1.15 mT for only 20 minutes.
As a result, The DNA synthesis phase of the cell cycle increased 24 hours after the exposure, and cell count increased somewhat after 36 hours, meaning that the EMFs promoted cell proliferation.
Increased cell counts were observed in three culture dishes with initial cell counts of 9000, 13500, and 18000.
Increase in DNA Synthesis Phase
The percentage of DNA synthesis phase increased by 7 percentage points due to the EMF exposure.
Increase in Cell Count
Zhong et al. 2012
Mouse bone marrow stromal cells, which have the ability to differentiate into osteoblasts and other cells, were exposed to 50 Hz ELF-EMFs with a strength of 0.5 mT for 8 hours a day for 12 days.
As a result, cell count increased during the exposure period, and the proliferation index increased on day 7 of the exposure, meaning that the EMFs promoted cell proliferation.
In addition, osteoblasts increased during the exposure period, meaning that the EMFs also promoted cell differentiation.
Increase in Cell Count
The cell counts increased by 50-60% due to the EMF exposure.
Increase in Proliferation Index
The proliferation index increased by 90% due to the EMF exposure.
Increase in Osteoblasts
The marker for osteoblasts increased by 50-70% due to the EMF exposure.
Phillips et al. 1986
Human colon cancer cells were exposed to 50Hz ELF magnetic fields with a strength of 50 μT, or 50Hz ELF electric fields with a strength of 300 mA/m2, or ELF-EMF of a combination of the two, for 24 hours.
As a result, two weeks after exposure, colony formation of the cancer cells increased, especially depending on the magnetic field component.
Increase in Colony Formation
The colony formation of cancer cells increased 2-fold with electric fields, 14-fold with magnetic fields, and 24-fold with electric and magnetic fields.
That's all for the presentation of the studies.
We have confirmed that EMFs can indeed promote cell proliferation.